Courtesy: Jiri Sedlacek/Wikipeida
Using reclaimed water as a makeup supply for cooling tower systems has become a popular option for reducing impacts on local water resources, but it comes with its own challenges because of its unique chemistry. Use of a novel terpolymer can greatly reduce the operational costs involved.
Mounting trends in global water stresses are creating an urgent need for water conservation strategies across industries that depend on large volumes of reliable supplies. The power industry, one of the heaviest users in the U.S., requires tremendous amounts of water for use in cooling towers. Based on heightened concerns related to water scarcity, greater competition for limited freshwater resources, and driven by the need to minimize risks related to water supply disruptions, the power industry is increasingly being forced to turn to non-traditional resources.
One option is treated municipal wastewater, or reclaimed water, which can help relieve strains on diminishing freshwater supplies. In recent years, the use of reclaimed water as a makeup water source in power plants has become more prevalent, especially in drought-prone areas such as the Southwest and water-challenged regions like Florida. Indeed, reclaimed water is becoming the standard for new power plant construction as well as a viable alternative makeup water source for existing facilities that are looking to expand production. (For one recent example of such a retrofit, see Tampa Electric Co.’s Polk Power Station, a 2015 POWERTop Plant.)
However, the use of reclaimed water for cooling tower applications represents a dramatic departure from freshwater. Not unexpectedly, reclaimed water presents a number of operational risks and challenges related to the propensity for elevated and variable concentrations of phosphate, ammonia, organics, chlorine, select metals, and other potentially problematic constituents. In particular, the phosphate concentration is a key factor that can dictate process requirements and operational parameters of the cooling water system in large part due to its tendency to form scaling species such as calcium phosphate.
High-phosphate reclaimed water can also exert additional challenges on cooling water chemistries, dictating the need for pretreatment/treatment steps, limiting attainable cycles, and impacting overall performance. In some cases, the level of phosphate concentration is a primary factor in determining whether a wastewater source is a suitable makeup water option.
This analysis explores the key drivers, risks and challenges, and treatment approaches in using reclaimed water as a cooling tower makeup supply in power plants. Particular emphasis is placed on an advanced chemical treatment program that allows for reclaimed water with variable and elevated phosphate levels to be used in a cooling tower system. This chemical approach—a novel terpolymer—has demonstrated that high-concentration phosphate waters can be successfully maintained in a cooling tower without the need for additional unit operations and pretreatment/treatment steps, thus eliminating capital- and operational-intensive processes and increasing the applicability of available reclaimed waters that can be used as a makeup water supply.
Reclaimed Water Characteristics
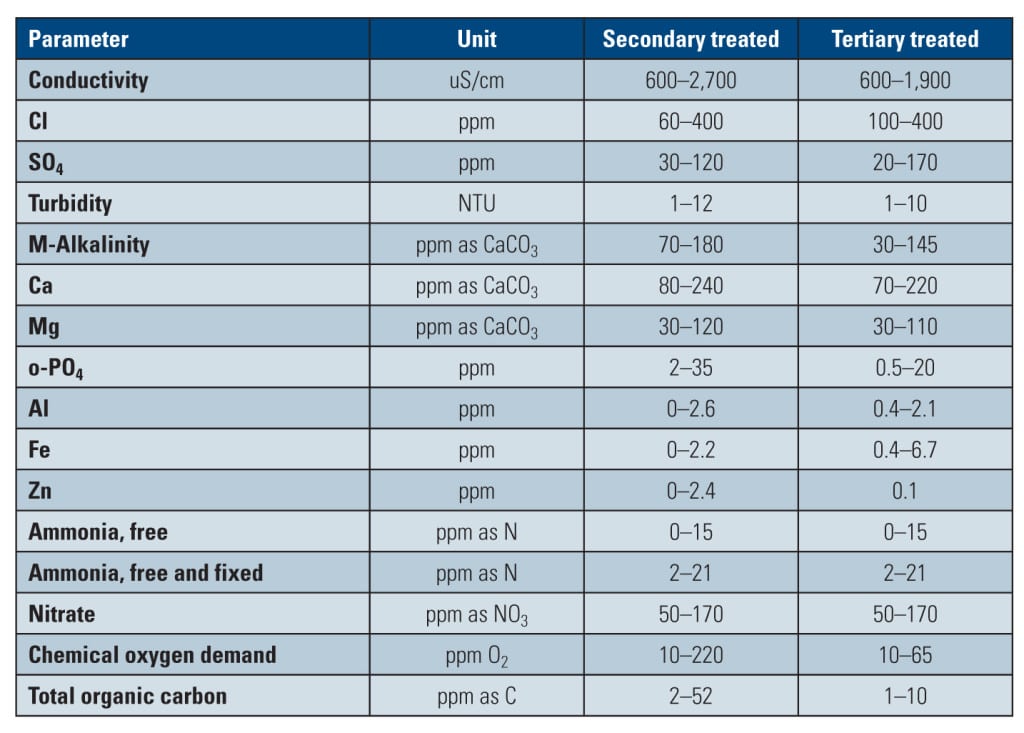
“Reclaimed water” refers to municipal wastewater that has been treated to meet specific water quality criteria with the intent of being reused for a range of beneficial purposes. Widely available in sufficient volumes across the U.S., reclaimed water can be treated to secondary or tertiary levels, as well as to the minimum requirements as specified by Title 22 of the California Administrative Code. The chemistries of these varying reclaimed waters are shown in Table 1.
Secondary treatment includes oxidation and settlement processes to remove organics such as oil and grease, while tertiary treatment adds precipitation and filtration steps for addressing scale-forming species. Reclaimed water treated to Title 22 standards—known as “Title 22 Water”—incorporates final chlorination to meet specific disinfection requirements.
Municipal effluent treatment is driven by Clean Water Act regulations, which set discharge limits on a range of contaminants and pollutants. Wastewater treated to at least a secondary treatment level includes standards that are placed on fecal coliform, five-day biochemical oxygen demand, residual chlorine, total suspended solids, and pH parameters. However, what’s important to note is that secondary treatment does not set limits or requirements on hardness, phosphate, or total dissolved solids—parameters that are very important for cooling water treatment.
Still, with only about 6% of the total available municipal wastewater volume presently reused in the U.S., the opportunity to utilize reclaimed water as a makeup water source for cooling towers is substantial.
Challenges and Risks
In evaluating the feasibility of using reclaimed water as a cooling tower makeup water source, a range of unique challenges and risks must be considered and addressed. Even though governmental regulations prescribe water quality parameters, not all reclaimed water is created equal. Depending on the source and the pretreatment methods used, the concentrations of problematic contaminants—such as iron, phosphate, and ammonia—can vary significantly. Moreover, each water source can have different concentrations of hardness, silica, and other contaminants that may limit the maximum concentration ratio (cycles of concentration) at which cooling towers may safely operate.
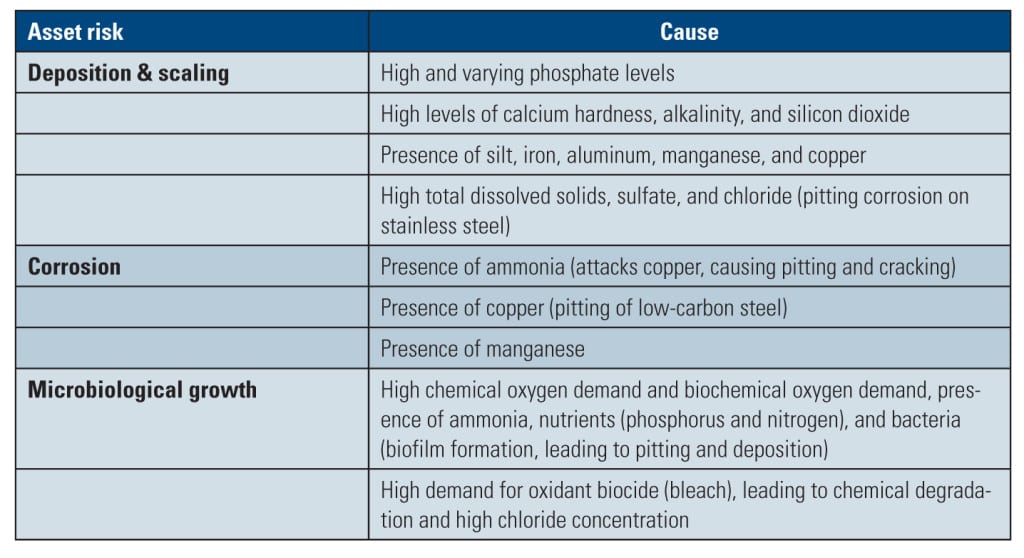
The chemistry of reclaimed water can also vary significantly over time, with fluctuating levels of problematic contaminants that increase the tendency for deposition/scaling, corrosion, and microbiological growth—all of which can directly damage cooling water system components or impede the ability of traditional cooling water treatment chemicals to function effectively. The risks (and their causes) associated with the use of reclaimed water for tower cooling are summarized in Table 2.
Of particular concern is the possibility of elevated phosphate concentrations, which present a severe risk of calcium phosphate deposition in the cooling system. Reclaimed water can also carry high amounts of fine particles and heavy metals/metals, which increase the tendency for scale formation and fouling. The presence of copper, even at a very low level—as low as 0.1 ppm—can cause significant pitting corrosion of low-carbon steel. Manganese may create special concerns with respect to deposition and subsequent severe pitting corrosion on metals such as stainless steel. At concentrations higher than 0.2 ppm, manganese deposition is very likely in the absence of an effective treatment. Reclaimed water at detectable manganese concentrations (0.02 ppm) may also cause manganese deposition problems.
Another significant challenge includes the potential for microbiological activity in the circulating water system. In using reclaimed water, large populations and numerous types of microorganisms are carried to the cooling tower. With high levels of chemical oxygen demand and total organic carbon loading, including the presence of ammonia, nitrogen, and phosphate, cycled cooling water is an ideal environment for microbiological growth.
As such, effective microbiological control is extremely important for maintaining efficient and reliable equipment operation. If left unchecked, microorganisms can rapidly accumulate and form biofilms and algae on tower fill, decking, transfer piping, and heat transfer surfaces. This can diminish heat transfer efficiency and promote under-deposit corrosion. Some microbes also pose significant health concerns.
Treatment Approaches
A cooling program that utilizes reclaimed water as a makeup water supply must be resilient against the inherent risks and able to cope with the extreme variability of the water chemistry. Facilities also need to be prepared to adapt to a stressed water condition and implement a robust treatment approach that includes close monitoring and an effective control strategy that takes into consideration the unique contaminant challenges of the reclaimed water supply as well as the specific materials of construction used in the cooling water system.
To address water hardness, a softening process can be utilized to precipitate species such as phosphate, calcium, magnesium, and silica in clarifiers. If phosphate levels need to be further lowered in secondary-treated reclaimed water, a biological phosphorous removal process can be installed. When excess nutrients—such as ammonia nitrogen—become a concern, an advanced biological nitrification process can be added to remove the ammonia-nitrogen by oxidizing it to nitrate-nitrogen. While these supplemental processes can generate adequate water, they also add significant capital and operating costs.
Likewise, side-stream processing of recirculating cooling water is able to successfully remove contaminants from cooling water and can be accomplished through processes such as membrane filtration (such as reverse osmosis) or by using softening (such as cold lime softening using clarifiers). However, these processes are also very capital intensive and carry high operating expenses because of requirements for ancillary unit operations such as dewatering, solids management, or brine disposal.
Chemical treatment programs represent another option for dealing with highly stressed reclaimed water and protecting cooling systems from corrosion and deposition. Such an approach can allow power plants to utilize low-quality waters—including those with elevated phosphate levels—without the need for costly operations, such as additional makeup water softening and side stream softening. Moreover, in addition to eliminating the investment that would otherwise be required for installing additional water pretreatment/treatment processes, chemical treatment programs also offer supplementary benefits related to more streamlined and simplified operations, reducing operating costs further. An example of a successful chemical treatment program for addressing high-phosphate, secondary-treated reclaimed water is provided next.
Using a Terpolymer to Treat High Phosphate Levels
At a 1,800-MW combined cycle natural gas power plant in the southern U.S., a novel stress-tolerant terpolymer (STP) was employed to prevent calcium phosphate deposition in a cooling tower system that uses highly stressed reclaimed water as a makeup water source. The use of STP was able to successfully eliminate a capital- and operational-intensive side stream softening process that was previously used to treat recirculating cooling water.
Two mechanical draft-cooling towers—each of which comprises 11 cells, a high-efficient film fill, and a recirculation rate of approximately 212,000 gallons per minute—are utilized at the plant to meet cooling needs. A local municipal wastewater treatment facility supplies the plant with reclaimed water treated to a secondary level, which accounts for the entirety of the plant’s makeup water supply.
The reclaimed water contains an average 13 ppm ortho-phosphate (o-PO4) with a historical maximum of nearly 30 ppm o-PO4. Side stream softening units were originally installed in the cooling loop to reduce o-PO4, calcium, and magnesium concentrations so that the cooling tower could operate at seven cycles with o-PO4 controlled at about 25 ppm, calcium hardness at about 500 ppm as calcium carbonate, and pH at around 7.
However, operation of the side stream softening process was expensive, as lime, soda ash, ferric chloride, and polymer had to be added in order to reduce hardness. Acid was also required at the effluent to control post precipitation. The softening units also required ancillary unit operations including filtration, which demanded constant attention, and the solids that were generated required hauling for disposal.
In 2014, it was proposed that the plant could operate the cooling water systems by using STP and removing the side stream softening units (Figure 1). This approach promised significant operating expense reductions, greater operational simplicity, and better safety. Additionally, by eliminating the need for sludge disposal, costs could be reduced while improving the plant’s environmental footprint.
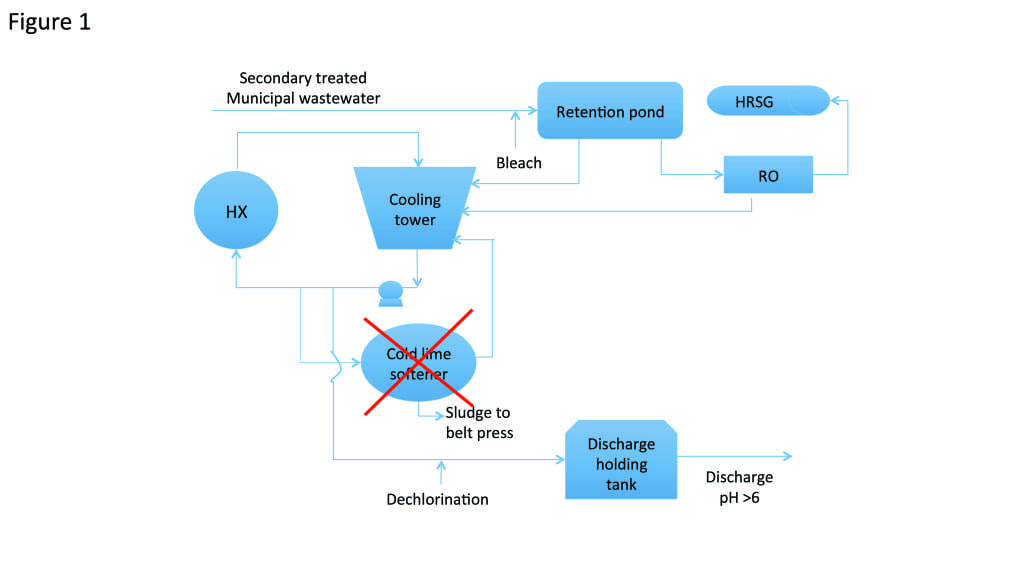
1. Sideways. This process schematic shows how one plant simplified its reclaimed water treatment process and eliminated side stream softening. Courtesy: GE
Without the softening process, it was clear the plant would face challenges related to the high concentration of phosphate, calcium hardness, and particulates present in the secondary-treated reclaimed water. The elevated phosphate concentration presented the extremely high risk of calcium phosphate deposition on condensers and tower fill, and pH excursions at such high concentrations could cause even more severe condenser deposition issues.
To evaluate the feasibility of using the STP, and enabling for cooling tower operation without the side stream softening process, comprehensive studies were performed addressing the inherent operational challenges. As part of these efforts, a mitigation plan was developed for high pH excursions so that deposition could be effectively prevented on the condensers in the event of a loss of acid feed.
A laboratory evaluation demonstrated superior performance of the STP for treating worst-case scenario phosphate concentration water, thus proving the feasibility to operate the cooling tower without a softening process under such conditions. Next, a test conducted in a pilot evaporative research tower employing the actual reclaimed water used at the plant showed that the designed program met all requirements for treating the reclaimed water without the side stream process.
Following these evaluations, a nine-month full-scale trial was completed in one cooling system using the STP while the other continued to operate as normal. The goal of the treatment program was to maintain equipment cleanliness and optimal performance. Bleach was added to control microbiological growth, and non-oxidant biocide and bio-dispersant were fed periodically to maintain surface cleanliness of the tower fill and condensers. Controlling pH within the designed range of 6.4–7.0 was a critical operating parameter to monitor, as high pH excursions without the softening process would significantly increase the chance of calcium phosphate deposition.
Guidelines were prepared for managing the plant’s cooling process without side stream softening, and key performance indicators—including condenser backpressure, condenser cleanliness, and water flow through plate and frame heat exchangers—were monitored online on an hourly basis. Basic wet chemistry tests on makeup and tower water were routinely performed, and full water analyses were conducted three times per week. Microbiological activity was monitored, and data related to water quality, plant operation, and condenser performance was uploaded to an online data management system for further analysis and calculations.
Results of the full-scale trial demonstrated that the STP treatment program maintained the cooling system in excellent condition. The o-PO4 in the makeup water cycled to 100% of what would be expected based on hydraulic cycles, indicating that the STP polymer was able to keep PO4 soluble even when the tower’s PO4 level reached 100 ppm. A delta PO4 target of less than 3 ppm was maintained with the average delta PO4 at approximately 1.2 ppm (Figure 2). Excellent deposition inhibition performance was also maintained throughout the trial with no signs of any scaling issues. Discharge pH levels did not exceed permit requirements, and at the end of the nine-month trial, a visual inspection and data review concluded that no condenser scaling had occurred.
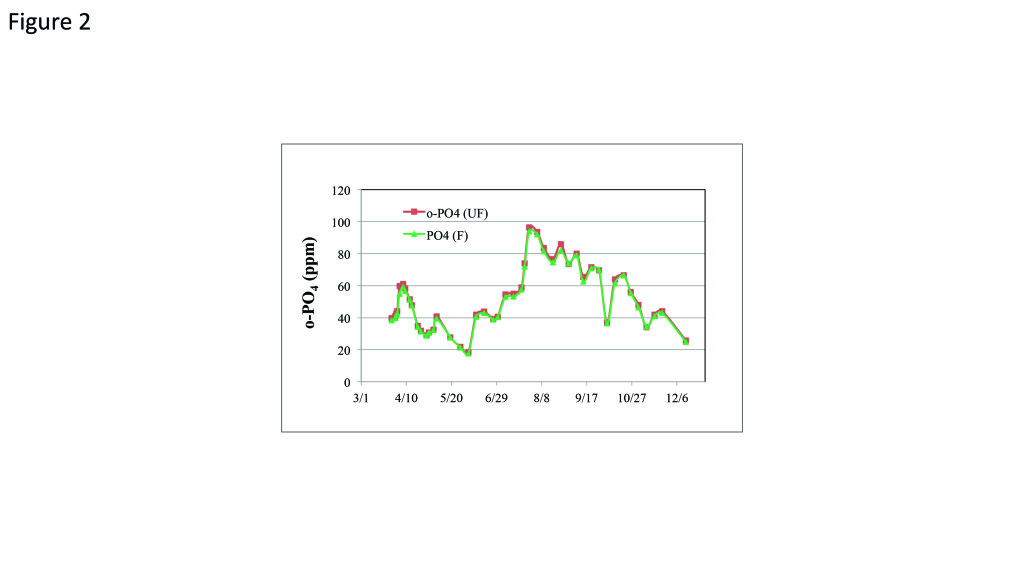
2. Unfiltered. Use of the stress-tolerant terpolymer kept ortho-phosphate (o-PO4) in the cooling tower within safe levels even without softening. Source: GE
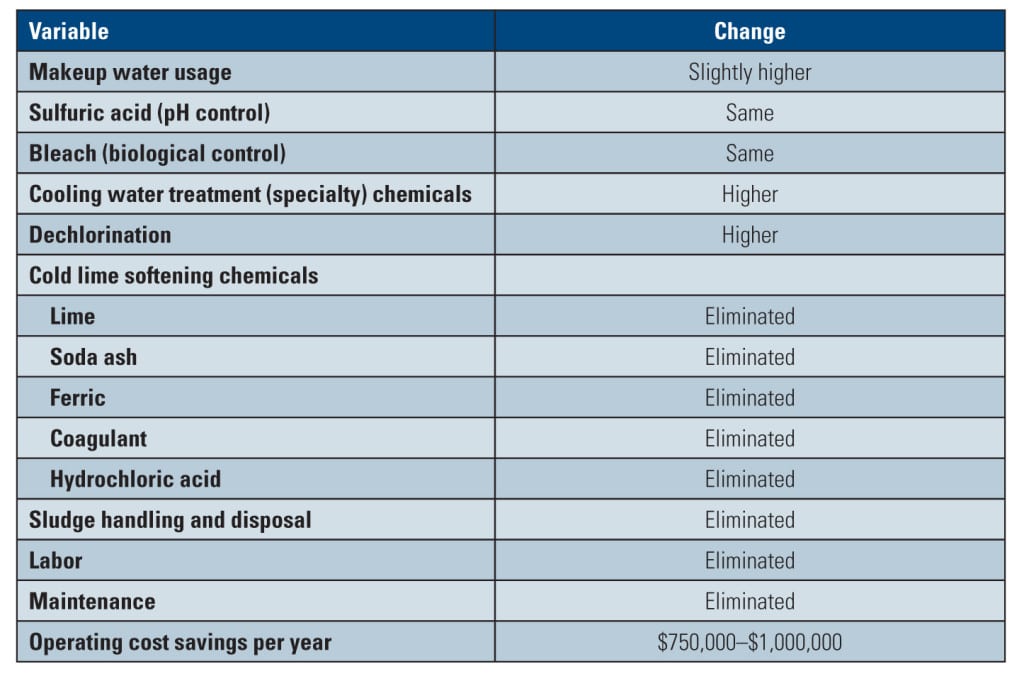
Removing the side stream softening process simplified the cooling system’s operation by eliminating chemicals and the need for sludge disposal. Labor and maintenance associated with softening were also eliminated. The resulting annual overall net operational savings were calculated to be $750,000 to $1,000,000 (Table 3).
Following the trial period, the plant continued with the STP treatment, and the second cooling system was converted to the new treatment program six months later, shutting down the other side stream softening process.
Savings and Reduced Complexity
By employing STP, such low-quality stressed water (with variable and high phosphate concentrations) may be treated without the added capital expenditure for softening. In these cases, the use of STP can lead to a capital cost avoidance for applicable new construction facilities as well as facilities that are considering expanding but do not have access to additional freshwater supplies. For example, for the power plant discussed in this paper, it is estimated that the associated installed capital cost for the cold lime softening process—including clarifiers, belt presses, and ancillary equipment—was $5 million to $10 million.
Reclaimed water offers tremendous potential as a viable and long-term supply option for power plant cooling applications. But as with any makeup water supply alternative, a range of built-in risks and challenges exist that must be addressed to achieve a successful implementation.
Several treatment solutions are available for managing the contaminant challenges associated with reclaimed water. As shown, the proper cooling tower treatment—including dosage, delivery, and control—can lead to the ability to treat harsh waters with elevated and varying phosphate levels without the need to invest in costly and more complicated processes. Moreover, in more overarching terms, a wider array of harsh reclaimed waters may indeed prove to be viable for use as cooling tower makeup water. ■
—Robert A. Hendel is a senior engineer–cooling, Caroline Sui is a senior research scientist, and Jeffrey Melzer is a water services technology leader with GE Water & Process Technologies.
https://www.powermag.com/using-reclaimed-water-power-plant-cooling-applications/?pagenum=1