Lubricant additive technology is a complicated business because it involves several different chemistries. Often, one additive can adversely interact with another additive as they both compete for the same area on substrate surfaces. This kind of interaction can lead to the canceling of the additives’ desired properties.
Conversely, the use of different additive chemistries can, at times, have a positive synergistic effect on performance. Therefore, understanding the complex chemistries and interactions of various lubricant additives is paramount to properly formulating a high-performance grease that fits an application’s requirements.
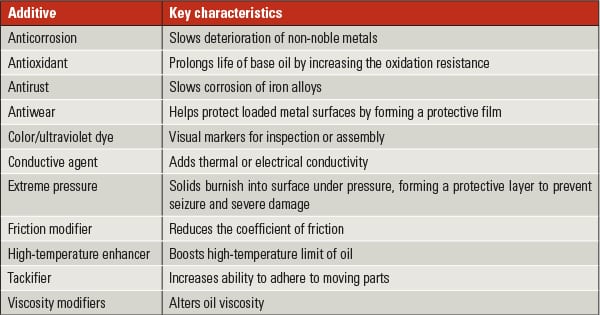
Some of the more important additives used in formulation greases are listed in Table 1.
 |
|
Table 1. What’s in your grease? This table shows characteristics of some common grease additives. Source: Nye Lubricants Inc. |
Antioxidants
Many synthetic lubricants, especially hydrocarbon-based lubricating oils, are susceptible to oxygen degradation. This oxidation process, which is initiated by the formation of reactive free radicals and peroxides, is the major cause of oil thickening and the formation of sludge and varnish in many applications.
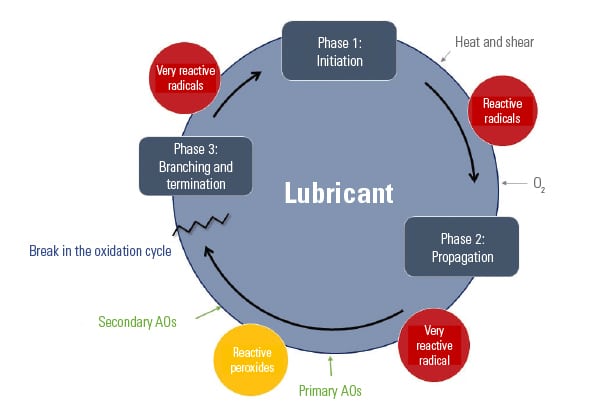
Destructive oxidation of oil can be described as a cyclical process involving initiation, propagation, and branching and termination (Figure 4). Unless the cycle is broken or terminated, oxidation will continue to occur until the oil or grease is no longer usable.
Antioxidants are additives designed to prolong the life of a lubricant by increasing the oxidative resistance of the base oil. Antioxidants allow lubricants to operate at higher temperatures than would otherwise be possible without them.
There are two types of antioxidants: primary and secondary. Primary antioxidants are typically comprised of aromatic amines and hindered phenolics. Secondary antioxidants are typically comprised of phosphites and certain sulfur-containing compounds, such as thioethers and thioesters. Each type of antioxidant performs a different function to inhibit oxidation.
The oxidation process begins with the initiation phase, when free radicals are formed. Primary antioxidants are “radical scavengers” that react quickly with the free radicals during the propagation phase, slowing down the degradation process by forming new radicals that are more stable.
Secondary antioxidants react with peroxides, which are often present as the lubricating oil reacts with oxygen. These antioxidants are responsible for breaking the cycle and preventing branching and further propagation. Most often, grease formulators will use a combination of primary and secondary antioxidants to maximize the protection of the oil against oxidative degradation.
Extreme Pressure and Antiwear
An expert at the Massachusetts Institute of Technology claims that high friction and wear results in the wastage of resources totaling more than 6% of the gross national product. Therefore, taking action to minimize the effects of friction and wear can save a lot of money.
There are several different types of mechanical wear mechanisms that can occur in a lubricating system, including adhesive wear, abrasive wear, pitting, and spalling. Wear preventative additives are a necessary component in lubricants intended for applications that are prone to such damage.
Wear preventative additives fall into two categories: extreme pressure agents and antiwear agents. Extreme pressure and antiwear additives both function by depositing a protective barrier via a reaction on the metal surface.
Antiwear additives are generally used under mild conditions of low loads and high speeds to reduce the rate of continuous and moderate wear. The additive helps coat the application surface to protect the metal from wear over time. Common examples of antiwear additives are triaryl phosphates and zinc dialkyldithiophosphate.
Extreme pressure additives are usually used under heavier loads, at high temperatures and low speeds to prevent catastrophic failure or seizing of the application. Common examples of extreme pressure additives are molybdenum disulfide, graphite, sulfurized olefins, and dialkyldithiocarbamate complexes.
Anticorrosion and Antirust
Corrosion is defined as the destructive alteration of metal by a chemical or electrochemical reaction between the metal and its environment, resulting in change and weakening of the metal’s properties. Corrosion is a process, not a property. All metals except the noble metals are unstable under atmospheric conditions, which allows them to be converted into their oxidized form. There are two primary types of corrosion: electrochemical corrosion and chemical corrosion.
Electrochemical corrosion involves the reaction of ferrous (iron-based) metal or its alloys in a two-step process in the presence of an electrolyte, most typically water or moisture. The result of this reaction is commonly known as rust.
Rust inhibitors can be included in a formulation to slow corrosion of iron alloys. Rust inhibitors function by physically adsorbing onto the metal surface, thus blocking the surface of the metal from the effects of water, acids, and air.
Chemical corrosion involves the attack of aggressive chemical species like acids, bases, and sulfur. This is often the result of the oxidation of hydrocarbons and sulfur-containing additives, or additive by-products, on a metal surface or metal oxide layer, resulting in the formation of ionic metallic or oxidized metallic compounds. Unlike electrochemical corrosion, chemical corrosion does not require an electrolyte, such as water, and can occur both in organic and aqueous environments.
Adding corrosion inhibitors to a lubricant will help slow the deterioration process in non-noble metals. The inhibitors form an inactive film on the metal surface by complexing with metallic ions at the surface. Some corrosion additives work by neutralizing corrosive acids formed from oil and additive degradation byproducts.
Lubricant Testing
There are several test methods used to measure the effectiveness of additive packages. To determine oxidative resistance, a pressure differential scanning calorimeter is most commonly used. The test measures the ability of an oil or grease to resist oxidation in a very demanding high-pressure oxygen-rich environment at elevated temperatures. The longer it takes for an exothermic reaction to occur, the better the oxidative stability.
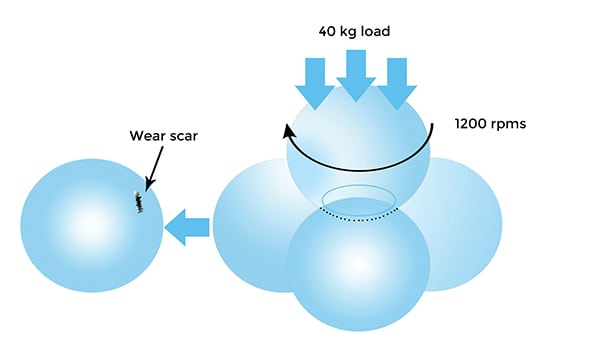
A common technique for measuring friction and wear is the four-ball wear test. In this test, a steel ball is rotated against three stationary, lubricated balls (Figure 5). Results are presented as wear scars on the stationary balls, which are measured and averaged, along with the coefficient of friction.
Another way to measure friction and wear is using a schwingung (oscillating), reibung (friction), verschleiž (wear) instrument. The method measures the physical interactions between a lubricant and two or more solid surfaces in either rotational or linear oscillatory motion.
The ability of a grease to prevent corrosion is most commonly tested using the copper corrosion test. In this test, a copper strip is submerged in grease and placed in an oven for 24 hours. The strip is then visually compared to a corrosion standard and given a rating (Figure 6). There are several other methods for measuring corrosion, including the fog spray test, corrosion rate evaluation procedure, the bearing corrosion test, and the EMCOR test.
Nye Lubricants’ research and development and applications laboratories have the capability to run all of these tests. Nye engineers can work with customers to tailor test methods to best simulate desired applications. This ensures that the lubricant chosen provides the protection that is needed. ■
—Anthony Grossi, PhD is director of technology at Nye Lubricants Inc.
https://www.powermag.com/all-about-lubricant-additives/?pagenum=3