The race is on to claim the title of "most efficient coal-fired power plant" on the planet. However, it’s tricky identifying finalists because of the widespread misuse of the term "efficiency" and all those nagging assumptions. Let’s first establish clear definitions and then identify the title contenders.
Most utilities will cover rising demand for electricity by building newer, higher efficiency plants and by adding energy efficiency and conservation (EEC) measures to their systems. EEC options include measures taken on the demand side (such as insulating residences, installing compact fluorescent lights, and advocating for building codes that promote more energy efficient construction practices), demand shifting (industrial process changes and energy storage), and even deploying strategically located distributed generation assets. Another approach is one used by many industrial facilities with a large appetite for electricity and thermal energy: Build a cogeneration plant that simultaneously produces cost-effective electricity, reduces expensive demand charges, and makes the needed thermal energy by recovering waste heat from the prime mover. The most common prime movers are gas-fired combustion turbines or reciprocating engines.
CHP Has a Long History
Cogeneration or combined heat and power (CHP) systems are far from being a new technology (Figure 1). In fact, many of the early electricity plants built at the turn of the prior century adapted their electricity production to systems that coincidentally recovered thermal energy from process heat that would otherwise be wasted. These small cogeneration systems were custom designed for a particular factory, and those designs worked well even without a grid to provide backup power. Fuels, at that time, were principally coal and oil. By the 1930s, the transmission grid began to extend beyond large population centers to provide reliable and relatively inexpensive electricity to the masses. By the end of the World War II, those early cogeneration plants could not economically compete with central station plants, so most were soon shuttered.

1. Waste not. A combined heat and power plant recovers waste heat to produce thermal energy, reducing overall consumption of fuel and reducing air emissions (compared to burning fuel to generate electricity and heat). This technology has been used in industrial and other settings for over a century. Source: EPA
Rising prices for oil and natural gas in the 1970s pushed many U.S. industries to reconsider onsite cogeneration of electricity and thermal energy to reduce their internal energy costs. The Public Utilities Regulatory Policies Act (PURPA), signed into law in 1978, encouraged a return to CHP as a national imperative. PURPA, since repealed as part of the Energy Policy Act of 2005, required utilities to interconnect "qualified" cogeneration plants owned by non-utility companies that met a certain threshold "efficiency." PURPA may have lost favor in the power industry, but it did give birth to today’s independent power and merchant energy businesses.
European utilities learned the value of CHP systems earlier than the U.S. and routinely constructed power plants that double as thermal energy suppliers to local cities and towns. Utility-scale district heating (and cooling) is the norm in Europe but remains an oddity in the U.S., the ConEdison district steam system in Manhattan (which supplies heat, hot water, and air conditioning to 1,800 customers via 105 miles of mains and pipes) being the most notable exception.
Another difference: Most European CHP plants were built by government-owned utilities committed to district heating — much different than the U.S. utility model. A good example of a modern CHP plant is POWER’s 2009 Marmaduke Award winner. Den Haag (The Hague) power plant (profiled in the August 2009 issue) generates thermal energy from two gas turbines for the city’s downtown district heating system. Today, thousands of publicly and privately owned CHP plants in Europe produce reliable and economic electricity and thermal energy for their customers.
"Efficiency" — A Much-Misunderstood Term
Several readers have recently suggested that our nation is missing the EEC boat by not "retrofitting" existing steam power plants to operate in CHP mode. These readers cite the "low" thermal efficiency (around 32%) of our nation’s coal-fired plants and the ease with which their efficiency could be increased to 80% or even 90% by recovering the heat energy rejected to the condensing water. What usually follows is a reference to an equipment supplier’s advertisement citing the stats on a new cogeneration project or to a European plant that boasts of 85% efficiency. Others have referenced the U.S. Environmental Protection Agency’s (EPA’s) Combined Heat and Power Partnership website (www.epa.gov/chp), which states that achievable plant efficiencies range from 70% to 80% for a diesel engine to 70% to 75% for a combustion turbine (Figure 2). The website goes on to say, "Typical CHP applications have coincident power and thermal demands that must be met. It is reasonable, therefore, to consider the values of power and thermal output from a CHP system to be equal in many situations."
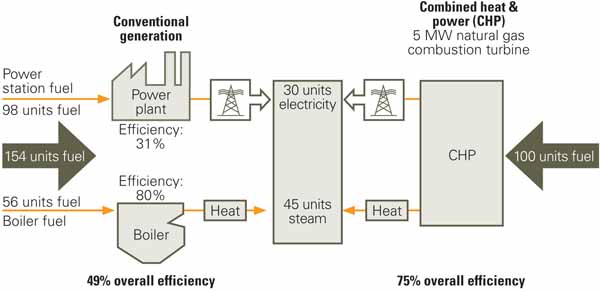
2. Missed the memo. The EPA’s notes to this example of a typical CHP system explain that, to produce 75 units of useful energy, the conventional generation or separate heat and power systems use 154 units of energy—98 for electricity production and 56 to produce heat— resulting in an overall efficiency of 49%. However, the CHP system needs only 100 units of energy to produce the 75 units of useful energy from a single fuel source, resulting in a total system efficiency of 75%. However, adding electricity and thermal energy together to produce an overall plant thermal “efficiency” is technically incorrect. The calculation is flawed because the “value” of the thermal energy is far less than the value of electricity. To do so is the technical equivalent of mixing your electricity apples with your thermal energy oranges. Some correctly note the difference and use the term ”fuel utilization factor” or “fuel utilization efficiency.” Source: EPA
Regrettably, many good engineers, equipment suppliers, and the EPA make a serious mistake by quoting these pseudo-thermal efficiency numbers, perhaps because they misunderstand the science behind the calculations.
Start with the Basics
The First Law of Thermodynamics is about energy accounting — energy out of a system equals the energy entering less the energy stored. The concept is simple. Most have experienced the dreaded low fuel light when driving to work. The amount of energy stored in your gas tank (gallons of gasoline) is the difference between what you have used and what you had when you last stopped and filled up the tank. One measure of a vehicle’s efficiency is the number of miles driven divided by the net gasoline used. The same quantitative analysis is used when calculating the thermal efficiency of a power plant: Measure the amount of electricity produced (less any losses) and then divide by the amount of fuel consumed to arrive at the plant’s thermal efficiency. This calculation method works and is universally accepted because the energy produced by the "system" is homogenous — it’s all electricity.
Others have incorrectly extrapolated the First Law efficiency calculation process to CHP systems by summing the electricity and the equivalent thermal energy produced by the system (see Figure 2) and then dividing by the fuel used to arrive at system "efficiency," frequently above 80%. This calculation method is misleading.
For example, if a power plant with a First Law efficiency of 50% sends half of its electricity to a heat pump with a coefficient of performance of four, then the First Law efficiency of this plant is 125%, clearly a useless term to compare power system performance. Using the First Law to make this calculation, though it is common today, is flawed because the "thermodynamic value" of the thermal energy is far less than that of electricity — you are mixing your electricity apples with your thermal energy oranges.
Upper Division Thermo
The Second Law of Thermodynamics is complicated and has many facets that are useful beyond the study of thermodynamics, including predicting the ultimate collapse of the universe and its beginning with a state of zero entropy. Whereas the First Law shows us how to "quantify" energy, the Second Law directs us to consider the "quality" of the energy in terms of what is defined as a "rational efficiency" when analyzing thermal systems.
For example, the First Law allows the design of a perpetual motion machine, while the Second Law prohibits its construction because every machine has losses, such as friction, that cannot be recovered.
Stated another way, the Second Law quantifies the ability of a thermodynamic system to perform work, and that ability to do work is directly related to the system operating temperatures. Higher temperatures mean higher system efficiency — higher steam temperatures increase efficiency in a steam plant, as do higher turbine inlet temperatures in a combustion turbine. Very low temperatures have a very limited ability to do useful work because the quality of the energy has been diluted.
Electricity is the most valuable form of energy available to us because it can be transmitted long distances, has a multitude of uses, and has a high energy density. Low-grade thermal energy has few uses, is not easily transported because of its very low energy density, and cannot be economically stored. The Second Law concludes that the thermodynamic value of thermal energy will always be less than that of electricity. Look back to your automobile for another analogy. The engine’s jacket water can efficiently provide the low-grade thermal energy to heat the occupants but is useless to provide the horsepower needed to move the vehicle forward.
This aspect of energy "quality" is missing when the First Law is used to calculate the "efficiency" of a CHP system. An example: Which would you rather have: A million Btu-equivalents of electricity available at your wall outlet or a million Btu of tepid bathwater? The First Law (quantity of energy) tells us they are equivalent and that you should be happy with either form of energy. The Second Law (quality of energy) says the value of the electricity is higher because of its superior ability to do useful work.
Widely Misused Terms
These thermo fundamentals have been universally ignored on a grand scale and are a stumbling block when identifying top-performing plants.
Vattenfall is one of the largest utilities in Europe and 100% owned by the Swedish state. Vattenfall is also the largest producer of thermal energy used for district heating in Europe and is dedicated to high-efficiency coal-fired power generation. One of its most advanced district heating plants in Europe is the Nordjylland Power Station, located in Denmark’s North Jutland region, near Aalborg (Figure 3). Vattenfall states on its web site that its ultrasupercritical (USC) Nordjylland Unit 3 is "the world’s most efficient coal-fired CHP plant." The plant sells its entire electricity production on the Nordic Power Exchange and more than 90% of its heat production to Aalborg District Heating Supply.

3. Efficient but expensive. Nordjylland Power Station’s USC Unit 3 is called the most efficient coal-fired CHP plant in the world by its owner, Vattenfall. A total of six supercritical and two ultrasupercritical plants call Denmark home. Each is configured to supply thermal energy for district heating systems. Even so, Denmark also has the highest household electricity prices in the EU, with retail rates surpassing $0.32/kWh, according to the U.S. Energy Information Administration. Courtesy: Vattenfall
Vattenfall makes its case this way: "Block [Unit] 3 has the world record in the use of fuel for coal blocks. But an efficiency of up to 91 percent by combined production and 47 percent [Editor: LHV-basis, 44.9% HHV — see the "Watch Your Terminology" sidebar] by clean electricity production using fuel about 20 percent better than older coal-fired plants." Later, Vattenfall goes on to state that "this is a world’s record that remains unbeaten." The 411-MW (384 MW net) Block 3 uses low-pressure turbine extraction steam to produce district heating water at 80C to 90C (176 F to 194F) by using a condensing heater. The double reheat USC plant fires bituminous coals sourced on the world’s markets.
Given the earlier discussion, equating electricity production with the equivalent amount of low-temperature hot water is incongruous with a claim that the plant efficiency more than doubles when in CHP mode (47% without district heat and 91% when steam is extracted from the low-pressure stages of the steam turbine to heat water). Vattenfall arrives at its claimed 91% efficiency by adding the energy equivalent of the electricity with that of the low-temperature hot water and then dividing by the fuel burned.
Here’s an example of how efficiency numbers vary. Assume a large power plant operates at 38% thermal efficiency. In addition, assume that this same plant produces about 40% usable heat (as a percentage of fuel input), much as Nordjylland Unit 3 does. Using these assumptions, the First Law efficiency is quickly calculated as 78%, whereas the rational efficiency calculated using the Second Law is 55%. The difference in the calculations lies with a correction made to the thermal energy based on the ability of the thermal energy to do useful work. Similarly, your gas-fired water heater has an advertised efficiency of 90% (First Law) but actually has a rational efficiency (Second Law) of only about 18%. The rational efficiency of a power plant that only produces electricity is the same as the First Law efficiency. Also, by definition, the rational efficiency will always be less than 100%, unlike the First Law.
Similarly, the owner of a small, inefficient gas-fired reciprocating engine can produce some electricity and recover the jacket water, lube oil, and exhaust gas energy to produce low-temperature hot water and a CHP "efficiency" in the high 90s while its rational efficiency is about 40%.
PURPA also recognized the difference in the value of electricity and thermal energy. The equation used to calculate the "PURPA efficiency" of a CHP plant was to add the electricity generated to one-half (an arbitrary factor to be sure) of the equivalent thermal energy delivered to the process and then divide by the amount of fuel used. In fact, the EPA’s technical support document (TSD) prepared supporting the Clean Air Interstate Rule recognized the problem with very high plant efficiencies claimed by owners. Regulators recognized that certain CHP plants should be exempted if they met a minimum efficiency standard. The EPA first proposed using the PURPA 42.5% efficiency threshold regardless of the fuel in order to prevent units with very low efficiency from claiming the CHP exception. The TSD noted that, "Without a minimum efficiency standard, a potential loop hole would exist for units to claim the exemption by sending a nominal or insignificant amount of thermal energy to a process." This situation occurs when a low-efficiency combustion turbine (and consequently high exhaust temperatures and higher emissions) is configured to produce very large quantities of low-quality thermal energy and thus sneak in under the standard.
Let’s be clear — the efficiency calculation methods defined by the laws of thermodynamics have little to do with the actual operating economics of any particular CHP system. In fact, CHP systems usually have exceptional economics as well as other tangible benefits to society. Just be aware of exaggerated claims of plant thermal efficiency that are extraordinarily higher than those of the prime mover (typically a gas turbine or engine and steam turbine) in the plant. Chances are the plant owners and equipment suppliers have incorrectly used the First Law of Thermodynamics to further their marketing programs or because they wish to assert bragging rights for the world’s most efficient this or that plant.
To enable more rational plant comparisons and promote sound science, I propose that we, as an industry, correct our bad habit of playing fast and loose with the laws of thermodynamics and use the Second Law or rational efficiency as the proper approach to calculating plant efficiency.
—Dr. Robert Peltier, PE is editor-in-chief of POWER
https://www.powermag.com/plant-efficiency-begin-with-the-right-definitions/?pagenum=4