Water treatment programs for boilers and heat-recovery steam generator (HRSG) units appropriately focus on chemistry controls during normal operation. However, rates of corrosion product transport and deposition can be much greater in boilers and HRSG units during start-up than during routine operation. For that reason, in addition to standard programs for monitoring and control of corrosion product transport, deposition, and underdeposit corrosion, consider adding contingency plans for boiler water chemistry holds in the start-up process.
A number of years ago a member of our organization visited a power plant to conduct a water treatment practice review. At the time, iron deposits on the turbine and significant iron in the blowdown samples indicated that the inventory of iron oxides in the steam cycle and iron oxide transport rates to the boiler and turbine were excessive. A procedure of blowing down the boiler during start-up to reduce the inventory of iron oxides was recommended as a remedial measure. This type of procedure has been commonly recommended at other facilities following chemical cleanings and occasionally in response to upsets resulting in excessive suspended solids in the boiler water.
Station personnel observed a number of start-ups and developed a start-up curve of iron versus pressure. The original curve extended from 500 psig to 2,700 psig (3.4 to 18.6 MPa) with iron concentrations of 2,650 ppb to 25 ppb (as Fe3O4) in the boiler water. It is our understanding that these iron concentrations were found to be essentially the pseudo-equilibrium levels that were achieved in a reasonable amount of time as a result of normal start-up practices.
In 2007 we were contacted again by this client to perform a steam/water cycle chemistry review. The station continued to utilize the start-up curve for boiler water iron levels. Station staff believed that their iron transport was well controlled during normal operation and start-ups, but they wanted an independent evaluation of the chemistry program.
Although station personnel monitored iron content in the boiler water during start-ups, they were not performing routine iron testing of the feedwater samples. Ordinarily, waterside deposits can be removed by chemical cleaning before failures occur. Deposits can lead to high temperatures and blistering (Figure 1), high and cyclic temperatures that lead to creep and thermal fatigue cracking and fireside corrosion (Figure 2), underdeposit concentration mechanisms and underdeposit corrosion (Figure 3), and boiler tube failures. In lieu of the feedwater corrosion product data, we assessed iron transport based on tube deposit accumulation rates.
1. Excessive boiler tube deposits and blistering. These preboiler corrosion products were deposited on the inner diameter surface near welds on the smooth tube portion. Deposits were up to seven times heavier on smooth tube than on rifled tube surfaces (~375 g/ft2) . The tube experienced short-term overheating (blistering) as well as other damage. Courtesy: Sheppard T. Powell Associates LLC

2. Heavy boiler tube deposits, overheating, fatigue, and fireside corrosion. The end view and outer diameter of the tube are shown in these images. The tube had heavy (146 g/ft2) internal deposits and had experienced long-term overheating, creep, thermal fatigue cracking, and fireside corrosion. Courtesy: Sheppard T. Powell Associates LLC
3. Boiler tube failure. The left side of the photograph shows the crack on the tube’s outer diameter. The right side shows an end view of the blistering and cracking from hydrogen damage. Heavy deposits formed on the tube surface, causing high temperatures, swelling, underdeposit concentration of trace acidic salts in the boiler water, underdeposit corrosion, hydrogen damage, and tube failures. Courtesy: Sheppard T. Powell Associates LLC
Since the most recent cleaning, the deposit accumulation rates for the two units had been 7.8 g/ft2/year and 8.9 g/ft2/year, and the total deposit loading during our visit was well above the level at which a chemical cleaning is recommended. When plants have accumulation rates over about 5 g/ft2/year, it is a sign of poorer-than-average control of iron transport and/or an unusually high localized deposition/corrosion mechanism. Plants with <1 to 2 g/ft2/year of deposit accumulation rate have good control.
For an earlier period with a higher deposit accumulation rate (10.7 g/ft2/year), iron transport rates were estimated to be about 26 ppb as iron in the feedwater (based on material removed and estimated total feedwater flow). For the more recent period, the iron transport rate was estimated to be 18 to 22 ppb as iron in the feedwater. The data also indicated that the rate of iron oxide transport during normal operation remains elevated.
Evaluation of Normal Operating Chemistry Program
The boiler operates at drum pressures up to 2,800 psig (19.3 MPa). With the exception of the condenser, the steam/water cycle was free of copper alloys. The cooling water is brackish with a low alkalinity. In the event of a condenser leak, boiler water becomes acidic.
The unit has full-flow, deep-bed condensate polishers operated beyond the ammonia break. The condensate/feedwater/boiler water chemical treatment program consists of ammonia and hydrazine additions (essentially AVT). Although the unit has polishers, they usually are bypassed during normal operation.
We suggested that the plant permanently stop the practice of bypassing the condensate polisher during normal operation to reduce iron and copper transport. Avoiding the bypass would significantly increase the frequency of polisher regenerations. The polishers are used in full-flow mode during start-ups.
The plant had excellent control of its feedwater cation conductivity (<0.15 µS/cm) and boiler water cation conductivity (the median was 0.31 to 0.38 µS/cm and start-up maximums were 1.80 µS/cm and 2.67 µS/cm for Units 1 and 2). Each unit normally only experiences one cold start and 10 to 12 hot starts per year.
It was expected that the elevated iron transport was more a reflection of iron transport during normal operation than during start-up activities. However, because the iron transport was already high, there was reason to be cautious about completely eliminating start-up boiler water iron limits.
The metallurgy and low feedwater cation conductivities enable eliminating the oxygen scavenger to reduce flow accelerated corrosion (FAC) and iron transport to the boiler. The risk of boiler tube corrosion due to moderately elevated oxygen levels with no oxygen scavenger should be minor. We advised that the benefits of lower iron transport during normal operation, lower deposit accumulation rates, decreased chemical cleaning frequency, and operation with lower deposit weights outweighed the potential disadvantages of not having an oxygen scavenger applied during start-ups or normal operation.
However, before eliminating the oxygen scavenger, we recommended that the plant perform an ammonium bromate cleaning of the high- and low-pressure feedwater system to remove the inventory of copper transported from the condenser during years of polisher bypass operation. Experience has found that copper deposits throughout the feedwater system with reducing treatment. Elimination of the reducing agent or an increase in oxygen subsequently has resulted in substantial copper transport to the boiler and/or turbine.
Feedwater Iron Limits
For electric utility drum-type boilers, iron limits in the feedwater are recommended to reduce the rate of deposition in the boiler (see sidebar for a list of boiler water chemistry best practices). Iron limits have been set at <10 to 100 ppb for preboiler cleanup (before applying fire to the boiler), <10 to100 ppb during start-up, and <5 to 10 ppb during normal operation. Steam/water cycles utilizing oxygenated treatment may achieve <0.5 to 3.0 ppb of iron oxide transport during normal operation.
Evaluation of Boiler Water Start-up Iron Limits
It’s common knowledge that large amounts of corrosion products, especially iron oxide, are transported to the boiler during start-up and that the largest spikes sometimes occur about one to three hours after an operational change. Increased transport of corrosion products has been observed nearly instantaneously after accounting for sample system delays. The increased transport is attributed to increasing temperatures (thermal expansion and stressing of oxide layers), sudden or increased flow (causing exfoliation, erosion, or FAC), or the reentrainment of sludge accumulated in reservoirs such as hotwells, heater shells, heater drip/drain tanks, or deaerator storage tanks. Sampling systems also experience deposition and subsequent reentrainment and provide nonrepresentative samples for a short period of time (typically 10 to 30 minutes) after a major transient.
Much of the corrosion material in the feedwater for conventional units is from the heater drains. Rather than returning the flow directly to the feedwater, heater drains should be routed to the condenser during start-up for units with condensate polishers. Heater drains should continue to be monitored until full load is reached because surges in flow at higher loads may result in appreciable corrosion product transport. For plants without polishers, diverting the heater drain condensate to the sewer is an option that should be considered during start-up.
For plants with full-flow condensate polishing, feedwater iron limits of 20 ppb have been effectively used during start-ups. At some plants with minimal preboiler cycle components or corrosion, lower limits (5 to 10 ppb) may be used. Facilities without deaerators or polishers generally need to minimize start-up time, as circulation or flushing is only useful if clean, noncorrosive water is used. Therefore, these facilities may need to utilize much less stringent limits.
The extent of corrosion product transport during start-up is largely dependent upon lay-up practices. Many power plants have noted that during start-up after long outages (such as those required for turbine overhauls) greater-than-normal amounts of suspended material are present in the boiler water. During long maintenance outages, nitrogen blanketing is not performed, and greater corrosion product formation and transport results. However, in some units even an overnight outage can result in substantial corrosion product formation and transport. For units that start up five or more times per week, out-of-service oxidation and corrosion product transport associated with start-ups normally is the dominant source of boiler deposits.
Blowdown also can be used to reduce iron levels in drum-type boilers. Obviously, increasing the boiler blowdown during start-up reduces the amount of iron available to precipitate on waterwall tubes and reduces deposit accumulation in the boiler. (If there is less to deposit, there will be less deposits.)
ASME studies from 1967 to 1969 by Goldstein, Klein, Rice, and Burton demonstrated that the sudden addition or presence of a large amount of suspended iron and copper oxides actually increased the likelihood of departure from nucleate boiling (DNB) on heat transfer surfaces. DNB results in dramatic increases in tube metal temperatures and can lead to localized deposition, overheating, dryout, and underdeposit corrosion. Though deposits increase the potential for DNB, the DNB experienced was due to the presence of suspended matter rather than the formation of a deposit layer. DNB stopped once the heat flux and suspended matter were reduced, stable boiling was reestablished, and the heat flux was increased to the original level (the boiler was not cleaned between these tests).
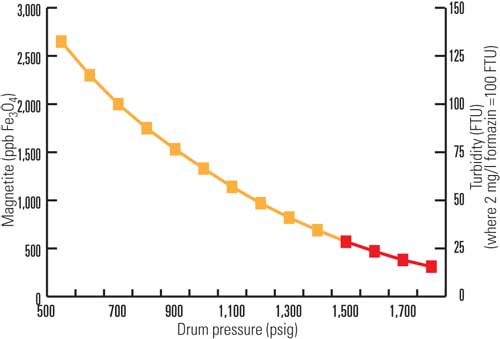
Once the boiler drum pressure reaches ~1,100 to 1,500 psig (7.6 to 10.3 MPa), the boiler water temperature is 8F to 48F (4C to 27C) above the temperature at which most of the iron oxides should tend to deposit (>550F or >288C). We also assessed the potential deposit accumulation in the boiler based on the boiler volume and operating conditions. Using an iron limit of 570 ppb at 1,500 psi, the mass of iron is calculated as only 0.32 lb (>150 grams). Assuming the plant experiences 14 start-ups per year, then the deposition of this quantity of iron oxide accounts for only about 45 lb of deposit after 10 years of operation. The mass balance approach indicates that the suspended iron during start-ups would need to exceed ~7 to 10 ppm (depending on the boiler start-up pressure and water density) to noticeably affect the total amount of iron removed during subsequent boiler chemical cleanings (>900 lb or >400 kg of magnetite in spent solvent).
One problem with this calculation method is the lack of representation of iron samples in the boiler water due to poor circulation during start-up and the tendency for suspended solids to sink or settle. Suspended matter will flow down to the lower headers but may not be carried back up to the steam drum due to the low rise rates in the waterwalls, or it may tend to settle in sloped tubes (nose tubes). Therefore, it is expected that iron levels can be much higher at the waterwall tube surface than in the continuous blowdown sample or downcomer sample.
Start-up iron curves have been used by various utilities with drum-type units for a number of years. Some plants stopped using the curve and did not report any noticeable increase in deposit accumulation or cleaning frequency. However, one plant that stopped using the curve reportedly had visibly black boiler water during start-ups, and the unit required chemical cleaning in just a few years.
The use of the interim boiler water iron control curve (see Figure 4 in the sidebar) only up to ~1,100 to 1,500 psig (7.6 to 10.3 MPa) and iron levels of 1,140 and 570 ppb were suggested based on the evaluation. Even though an extended version of this curve has been used for decades, it is considered an interim approach, because it is expected that higher iron levels could be maintained without significantly affecting unit integrity. The exact limit for suspended iron in a steam drum sample to limit waterwall tube deposits is unit-specific.
Because most drum-type boilers do not use boiler water start-up iron levels as a control parameter, the limits in Figure 4 are not required for most facilities during routine start-ups. However, a boiler water iron limit should be considered a backup to feedwater iron and copper limits. It may be useful to have this basic procedure as a contingency. The procedure can be used in instances where greater-than-usual levels of iron are present, such as at initial plant start-up, after chemical cleanings (especially if all residual sludge was not mechanically removed from lower headers after a chemical cleaning), after long outage periods, and in facilities with extremely dirty water for some other reason (if, for example, dirt was introduced during an outage or an extremely poor layup). This procedure can provide a safeguard against a horrendously dirty start-up. The rational basis for a boiler water iron limit is primarily to avoid DNB conditions. Ordinarily, if layup and start-up practices are optimized, the boiler water start-up holds should not need to be implemented.
Some have found that removing the fires from service at low pressure (100 to 500 psig, depending on boiler manufacturer recommendations and valve limitations) and performing bottom blows also significantly reduces levels of suspended corrosion products. This generally is only used in response to upset conditions.
Monitoring Iron in Feedwater
Most of this article focuses on iron, because it is the predominant corrosion product in virtually all steam/water cycles. Because iron is predominantly present as suspended oxides, exact analyses of grab samples requires acid-washed sampling apparatus and glassware and digestion before analysis. The traditional Millipore iron test was developed to meet these needs more than 40 years ago as part of the start-up of the first supercritical boiler in the U.S.
Tests found that nearly all of the iron was present in suspended form and was removed by filtration through a 0.45-micron filter. Some have used finer filters (0.20 to 0.22 micron) for slightly improved accuracy. The Millipore test probably will continue to be used for rapid assessment of grab samples during start-ups.
The Millipore test has the added benefit of indicating the presence of all types of suspended matter that may be present during start-up of a unit, including dirt, grit-blasting material, and copper and nickel oxides. One study found that zinc was completely soluble in condensate, copper can be present in suspended and/or soluble forms, and nickel can be present as mixtures of suspended oxides and soluble forms. Because there can be soluble forms of corrosion products, plants with a significant amount of nonferrous (copper, nickel, and zinc) alloys may need to perform additional tests to obtain an indication of the soluble component.
Traditional colorimetric methods for iron and copper analyses can be adapted to provide detection limits in the low-ppb range. Following digestion, graphite furnace atomic absorption (GFAA) spectrophotometry is still considered the benchmark analytical method by most power plant chemists. Digestion and inductively coupled plasma with a mass spectrometer (ICP-MS) is claimed to have equal or better detection limits, although the extremely low detection limits claimed by instrument suppliers are orders of magnitude lower than those offered by most commercial laboratories with ICP-MS instrumentation.
X-ray fluorescence (XRF) also has been developed for online, low-ppb monitoring of iron in feedwater. A study in a nuclear plant found that over 80% of the iron was transported into the unit in less than a two-hour period. XRF analyzes the suspended iron oxides that accumulate on a Millipore filter. The filter pad is changed every few days (Figure 5).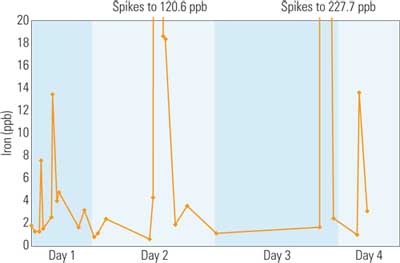
5. Online corrosion monitoring. This online corrosion products monitoring data, collected by an X-ray fluorescence detector for iron in a supercritical boiler, shows large spikes of iron oxides during plant start-up and cycling loads. The results cover a 65-hour, 42-minute period. Source: Sheppard T. Powell Associates LLC
For start-ups, other analytical methods are available for rapid assessment of suspended iron. Turbidimeters calibrated with 100 nephelometric turbidity units (NTU) = 2 mg/L of formazin were found to be directly proportional to suspended iron oxide (100 to 2,000 ppb = 5 to 100 NTU). This relationship corresponds to 20 ppb of iron oxide per NTU of turbidity, although the correlation is more variable at lower (<100 ppb) concentrations.
Indications of particulate matter also can be provided by particle monitors and particle counters (PCs). Particle monitors measure fluctuations in light intensity and provide a particle index value. There are two types of particle counters: those that rely on light blocking and those that rely on light scattering. Light-blocking PCs count the number of times shadows are cast by particles 1 or 2 µm to 100 µm. Light-scattering PCs determine the number of particles (down to 0.05 or 2.0 µm, depending on the instrument) based on the amount of light scattered. In addition to a total particle count, PC units usually can provide a breakdown of the number of particles in several particle size ranges, although total counts are often the most useful.
—Robert D. Bartholomew (rdb@stpa.com) is an associate with Sheppard T. Powell Associates LLC.