During the heyday of large utility steam plant construction, plant chemists and researchers learned many lessons regarding steam generation chemistry to prevent of corrosion, scale formation, and other mechanisms that caused equipment failures and outages.
When the author began his utility career in 1981, conventional wisdom said that any dissolved oxygen which entered the condensate/feedwater system of utility boilers was harmful. At that time, over 50% of the power produced in the U.S. came from coal.
Coal-fired units typically have complex condensate/feedwater networks with numerous feedwater heaters. The prevalent thinking was that any trace of dissolved oxygen (D.O) would cause corrosion, and indeed oxygen corrosion can be problematic in uncontrolled situations. Therefore, virtually all feedwater systems for high-pressure steam generators were equipped with a deaerator for dissolved gas removal. A properly operating deaerator can lower D.O. concentrations to 7 parts-per-billion (ppb).
However, any residual dissolved oxygen was still considered harmful, so chemical deaeration was a standard process at most plants. The workhorse for many years was hydrazine (N2H4), a reducing agent which reacts with oxygen as follows:
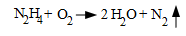
Also, a primary, and arguably the primary, benefit of hydrazine is that it will "passivate" oxidized areas of piping and tube materials as follows:
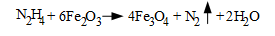
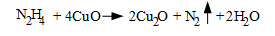
Fe3O4, magnetite, is the protective layer that forms on carbon steel when it is placed into service. Cu2O forms on copper alloys. (We will not discuss the latter chemistry further, as the focus is on the heat recovery steam generators (HRSGs) of combined-cycle units, although the principles also apply to many industrial steam generators. The condensate/feedwater systems in these boilers typically have no feedwater heaters or copper alloys.)
Hydrazine residuals were typically maintained at relatively low levels of perhaps 20 to 100 parts-per-billion (ppb). Oxygen scavenger treatment was coupled with feed of ammonia or an amine to maintain feedwater pH within a mildly alkaline range, now 9.1 to 9.3 for mixed-metallurgy feedwater systems but higher for all-ferrous systems.
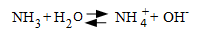
This program became known as all-volatile treatment reducing [AVT(R)].
Due to the suspected carcinogenic nature of hydrazine, alternative chemicals such as carbohydrazide, methyl ethyl ketoxime, and others gained popularity. Regardless, all still had the same purpose, to establish a reducing environment in the feedwater circuit, thus inhibiting oxidation of metal. The technique became a standard in the industry.
This changed in late 1986 when an elbow in the condensate system ruptured at the Surry Nuclear Power Station in Virginia. The failure 30 years ago caused four fatalities and tens of millions of dollars in repair costs and lost revenues. [1] According to a Dec. 22, 1986 news report in the Washington Post, workers were conducting maintenance Dec. 9 when, "with a pop heard throughout the sprawling plant, a pipe at Surry Unit 2 ruptured, twisting metal girders and dumping 30,000 gallons of superheated water in the turbine building. Eight men, including the four who later died, were injured."
Investigators learned from this accident and others that the reducing environment produced by oxygen scavenger feed results in single-phase flow-accelerated corrosion (FAC).
Single-phase FAC. Note the orange peel texture. Photo courtesy of ChemTreat.The attack occurs at flow disturbances such as elbows in feedwater piping and economizers, feedwater heater drains, locations downstream of valves and reducing fittings, attemperator piping, and, most notably for the combined-cycle industry, in low-pressure (LP) economizers and evaporators, and to a lesser extent in intermediate-pressure (IP) circuits. The effect of single-phase FAC is further illustrated below.

Photo of tube-wall thinning caused by single-phase FAC. Photo courtesy of ChemTreat.Metal loss occurs gradually until the remaining material at the affectedlocation can no longer withstand the process pressure, whereupon catastrophic failure occurs. The thinning is due to the combination of a reducing environment and localized fluid flow disturbances, which cause dissolution of ferrous ions (Fe+2) from the metal and metal oxide matrix.
EPRI research also showed that iron dissolution is greatly influenced by solution pH and temperature.
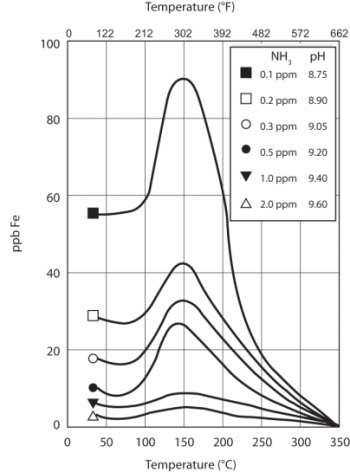
Carbon steel matrix dissolution as a function of pH and temperature. Source: Ref. 2.As the chart illustrates, corrosion reaches a maximum at 300o F. Thus, feedwater systems and HRSG low-pressure evaporators are particularly susceptible locations. Also note the influence of pH, as reflected by ammonia concentration, on the corrosion characteristics. As we shall see, this factor is quite important with regard to control of FAC.
Solutions to Single-Phase FAC
HRSGs by their very nature typically have many waterwall tubes with short-radius elbows. Thus, the HRSG contains numerous spots susceptible to single-phase FAC. A primary method to mitigate this attack is selection of proper feedwater treatment.
Over 40 years ago, researchers in Germany and later in Russia began using a program known as oxygenated treatment (OT) to minimize carbon steel corrosion and iron dissolution in supercritical steam generators. The key component of the program was deliberate injection of pure oxygen into the condensate/feedwater network to establish oxygen residuals of up to 300 ppb.
In very pure feedwater (cation conductivity, ≤ 0.15 µS/cm), the oxygen will intersperse and overlay magnetite to generate a tenacious and very insoluble film of ferric oxide hydrate (FeOOH). Now, OT is the preferred feedwater treatment for once-through utility steam generators around the world.
Although OT has been successfully applied to drum boilers, another program has evolved that is very popular for condensate/feedwater treatment in these steam generators. It is known as all-volatile treatment oxidizing [AVT(O)]. With AVT(O), the primary oxygen source comes from the (normally) small amount of air that enters the condenser via in-leakage. Note that OT or AVT(O) are not permissible for feedwater systems containing copper alloys, as the oxygen would simply be too corrosive to the metal. The following, therefore, focuses upon AVT(O) for all-ferrous systems.
Current AVT(O) guidelines are as follows:
• Condensate pump discharge (CPD) normal D.O. limit - ≤20 ppb
• Recommended feedwater pH range:
o FFLP HRSG 9.6-10.0
o SALP HRSG
• Feedwater D.O. concentration, 5-10 ppb
FFLP stands for feed forward low-pressure HRSG configuration, in which all of the feedwater is heated in the LP evaporator. SALP designates stand-alone low-pressure configuration, in which the feedwater is split between the LP, IP, and HP evaporators without LP pre-heating.
As with OT, the condensate in an AVT(O) programs must be quite pure to allow oxygen to generate the FeOOH protective layer rather than cause pitting. However, the cation conductivity upper limit with AVT(O) is a bit more relaxed at ≤ 0.2 µS/cm.
The amount of air in-leakage that establishes the “normal” condition of 20 ppb or less dissolved oxygen in the condensate is not a hard and fast value. The old rule of thumb for proper condenser conditions is a limit of 1 scfm (standard cubic feet per minute) of air in-leakage per 100 MW of capacity.
However, the author has worked with units in which the air in-leakage ratio was significantly higher, but where the condenser vacuum pumps had sufficient capacity to remove the gases. Quite often, a failure at the condenser shell or within auxiliary equipment may cause a sudden spike in dissolved oxygen concentration.
As contrasted to pure oxygen feed such as with OT, air in-leakage also allows carbon dioxide to be drawn into the condensate, which raises the conductivity. In such cases, plant personnel need to search for the leak or leaks and repair them promptly.
In some cases, the condenser may be so “tight” that air in-leakage does not provide enough oxygen to maintain the feedwater D.O. concentration at 5-10 ppb. A small, continuous feed of pure oxygen may be required to boost the concentration.
Chemical and Material Selection Techniques
As already noted, elevated pH has a beneficial effect in mitigating FAC. Thus, if tri-sodium phosphate (Na3PO4) or caustic are used for evaporator treatment, these compounds better help to maintain alkaline pH. Note though that in FFLP HRSGs, TSP or caustic are not permissible in the low-pressure drum, as the LP effluent serves as the source for the steam attemperators.
For new HRSGs, single-phase FAC control can also be addressed by materials selection. The addition of a small amount of chromium in the material at FAC-susceptible locations virtually eliminates the corrosion. A primary example is LP waterwall elbows. Fabrication of the elbows from 1¼ or 2¼ chrome alloy can provide great benefit. While this alloy addition adds some cost to the project, the materials are quite resistant to FAC.
Two-Phase FAC
Many steam generators, regardless of type, are susceptible to two-phase FAC. As the name implies, this corrosion mechanism occurs where water flashes to steam, resulting in a mixed-phase fluid. For conventional units, feedwater heater shells and heater drains are common locations for two-phase FAC, but this equipment is not common for HRSGs. However, deaerators also experience two-phase fluid flow.
As fluid flashes upon entering a deaerator, oxygen departs with the steam. Thus, the water that impinges upon metal surfaces does not maintain an oxidizing environment. Also, the pH of entrained water droplets within the steam is usually lower than the bulk water pH. The combination of these factors often initiates FAC.

Two-phase FAC in a deaerator. Photo courtesy of Tom Gilchrist.As with single-phase FAC, a method to combat two-phase FAC is fabrication of susceptible locations with chromium-containing steel. Again, this adds cost to the project.
FAC is a serious issue. In addition to the references included in this article, readers may also access the International Association for the Properties of Water and Steam. This organization offers free downloadable and technical information regarding power plant water/steam chemistry.
References
1. Guidelines for Controlling Flow-Accelerated Corrosion in Fossil and Combined Cycle Plants, EPRI, Palo Alto, Calif.: 2005. 1008082.
2. Buecker, B. and Shulder, S., “Power Plant Cycle Chemistry Fundamentals”, pre-workshop seminar at the 35th Annual Electric Utility Chemistry Workshop, June 2-4, 2015, Champaign, Ill.
3. K. Shields, [A report on the] “International Conference on Flow-accelerated Corrosion in Fossil, Combined-Cycle/HRSG and Renewable Energy Plants” at the 2013 spring meeting of the ASME Research Committee on Power Plant & Environmental Chemistry, April 15-17, Houston, Texas.
