Engineers and professional staff at industrial plants know that raw, intermediate, and final products may be highly corrosive to plant piping, heat exchangers, vessels, and other equipment, as well as to people.
However, corrosion can be just as serious on the water/steam side of the plant. In a series of articles we will examine some of the most important aspects of corrosion that are related to water and steam use in industrial plants and power generation facilities.
The primary metals that we will examine, especially in later parts of the series, are carbon steel, stainless steels, and copper alloys. We begin by outlining the most common corrosion mechanisms.
Corrosion Fundamentals
Corrosion of metals always involves an electrochemical process. But in the case of erosion-corrosion, mechanical influences are also present. Although many different types of corrosion have been discovered (and some have not yet been completely investigated), the following example illustrates the fundamental concepts of corrosion.
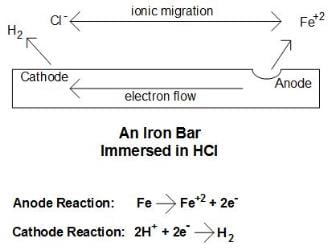
Consider a simple laboratory experiment of immersing an iron bar in a hydrochloric acid (HCl) solution. After a few days, corrosion spots will become plainly visible on the bar. The figure illustrates the corrosion mechanism.
(Click to enlarge.) Basic corrosion cell. Source: Author's construct.Three steps explain the basic process:
1. Each iron atom at the corrosion site gives up two electrons (oxidizes), and thus transforms from a zero oxidation state to a +2 oxidation state,
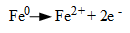 (Eq. 1.)
(Eq. 1.)
The Fe+2 (ferrous) ions migrate into the solution. This site is known as the anode.
2. The electrons flow through the metal to another site where they react with the hydrogen ions of the acid (reduction) to produce hydrogen gas,
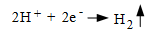 (Eq. 2.)
(Eq. 2.)
This reaction occurs at the cathode.
3. The anions from the acid, chloride ions (Cl-), and the ferrous ions migrate through solution to produce solvated ferrous chloride (FeCl2). What is evident from this example is that an electrical circuit is completed. Without this electrical circuit, corrosion does not occur.
Another fundamental cathodic reaction, and one that greatly influences water treatment is,
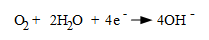 ( Eq. 3.)
( Eq. 3.)
This is the typical cathodic reaction in water containing dissolved oxygen. The hydroxyl ions combine with iron to form ferrous hydroxide, [Fe(OH)2], which further oxides to ferric hydroxide precipitate [Fe(OH)3]. Dehydrolysis of ferric hydroxide produces Fe2O3, which we commonly know as rust.
Many types of corrosion are known. Some, however, are more common than others. Eleven of the most important corrosion mechanisms concerning water/steam use are general corrosion, pitting , crevice corrosion, galvanic corrosion, erosion-corrosion (particularly flow accelerated corrosion), stress corrosion cracking, corrosion fatigue, intergranular corrosion, dealloying, exfoliation, and microbiologically-influenced-corrosion.
The following sections outline these mechanisms.
General Corrosion: General corrosion occurs when a metal is attacked uniformly by a corroding substance. The corrosion of active metals by acid is a prime example. Pipes, tubes, and other power plant materials are always designed with a general corrosion safety factor. Since general corrosion is spread over a wide area, metal integrity may remain solid for many years. Unfortunately, many corrosion mechanisms are not general in nature but are localized. These are much more serious.
Pitting: Pitting is a localized form of attack in which the corrosion penetrates down and through the metal. Pitting is an insidious mechanism and may cause failures even when the bulk of the material is still in good shape.
Several types of pitting are possible. One of the most common is under-deposit corrosion, which is a phenomenon that occurs in boilers and cooling water systems. Deposits in cooling water systems generate oxygen differential cells, in which the environment underneath the deposit becomes oxygen deficient in comparison to the bulk solution. This causes the tube metal below the deposit to become anodic to clean surfaces, where oxygen is in good supply.
Diagram showing a mechanism of localized corrosion developing on metal in a solution containing oxygen. Image source: WikipediaUnder-deposit corrosion is one of the primary reasons why it is so important to prevent fouling and scaling in water systems. Deposits in boilers may produce oxygen differential cells, or they may trap corrosive materials underneath that attack the boiler tubes directly.
Another form of pitting is an attack common to stainless steels. Stainless steels develop an oxide surface layer that protects the base metal. This oxide layer may be penetrated by impurities in the water. Chloride is a particularly notorious pitting agent, and it will attack some grades of stainless steel, including one of the most commonly used heat exchanger materials, 304 stainless. As the attack continues, the chloride concentration in the pit increases and the pH goes down, further exacerbating the problem.
Remember that although stainless steels are widely used throughout the power, chemical, and manufacturing industries for piping, vessels, heat exchangers, and other equipment, some people assume that stainless materials are bulletproof. This is a myth. If stainless steels are selected improperly or for the wrong application, corrosion failures can occur rapidly.
Crevice Corrosion: Crevice corrosion almost can be thought of as pitting, but in this case a mechanical factor, namely the physical joining together of two metals, leads to sites (crevices) that become oxygen depleted. These sites become anodic to the exposed metal surface and corrode in a similar mechanism to the under-deposit process.
Galvanic Corrosion: Galvanic corrosion occurs when two dissimilar metals are coupled together in a corrosive medium. The more passive of the two serves as a cathode and the more reactive becomes an anode. Galvanic corrosion can be especially severe when a small anode is coupled with a large cathode. One classic example is of copper plates that are connected by steel rivets in seawater service. Corrosion of the rivets can be intense. The reverse situation, namely copper rivets holding steel plates, is better from a corrosion standpoint, as a large anode is coupled to a small cathode. Although corrosion of the steel still occurs, metal loss is less severe.
Erosion Corrosion: Erosion-corrosion is a combination of physical and chemical attack. It occurs when a flowing stream erodes the outer layer of the metal exposing fresh metal to further corrosion. High fluid velocity or disturbances in flow patterns may initiate erosion-corrosion. A special form of erosion-corrosion, flow-accelerated-corrosion (FAC), has caused great concern in the power industry.
Stress Corrosion Cracking: Stress corrosion cracking (SCC) is another mechanical/chemical mechanism. The corrosion occurs at stress points in metals, which are attacked by a specific agent. The 304 and 316 stainless steels offer a good example. When placed under stress in a corrosive environment, such as a solution containing chlorides, the material will begin to corrode at the stress points. Turbine blades are prime locations for SCC, as they are constantly under stress.
Fatigue and Corrosion Fatigue: Breaking a wire by bending it back and forth repeatedly until it fails is an example of fatigue in which failure was caused by cyclical stress. Fatigue-related failures occur commonly at support locations in boilers where, as the tubes heat or cool, stress is placed at the point of attachment. Cyclical stresses are common in rotating machinery, and components may fail by fatigue in such locations. If fatigue stresses occur in an environment containing aggressive chemicals, fatigue failures are often accelerated. The chemical can penetrate metal at fatigue cracks to increase the cracking rate.
Intergranular corrosion attack in austenitic cold rolled stainless steel sheet. Image source: Creative CommonsIntergranular Corrosion: Intergranular corrosion occurs along the grain boundaries of metals. This attack is often set up by poor heat treatment of the metal. Improper heating or cooling of an alloy may occur during the fabrication process, or more often, when the material is being welded in the field. The extreme heat generated during welding can alter the crystalline structure of the alloy and reduce corrosion resistance. Many stainless steel failures have occurred due to poor welding, where the heat from the process causes chromium to combine with carbon into small globules. Chromium carbide precipitation reduces chromium’s ability to form a protective oxide layer on the steel surface and opens the way for corrosion.
Dealloying: Dealloying is the selective leaching of one metal from an alloy. Two of the most common forms are dezincification and denickelification of Admiralty metal and copper-nickel alloys, respectively. Although debate exists as to the exact corrosion mechanism, the results are similar: The zinc or nickel departs, leaving a spongy copper mass behind.
Exfoliation: Exfoliation is a readily observable phenomenon as flakes of metal appear on the pipe or tube surface. These flakes will often wash downstream and foul other areas of the steam generating system. Exfoliation may be the result of mechanical factors more than chemical attack. Exfoliation is commonly observed in high-temperature tubes and piping, where the high temperatures generate thick oxide layers. Cyclic operation places mechanical stress on the deposits, and eventually they begin to break loose and transport elsewhere.
Microbiologically Influenced Corrosion: Microbiologically-influenced corrosion, or MIC for short, is a common problem in cooling water systems. Microbes not only form deposits that may cause under-deposit corrosion, but many organisms also generate corrosive chemicals via metabolic processes. Typically, if microbes are allowed to attach to a metal surface and form a colony, the organisms secrete protective slimes for protection. While organisms near the surface may be aerobic because oxygen is present in the cooling water, the colonies underneath consist of anaerobic bacteria. These organisms live by using minerals in the water, and the byproduct of their metabolic biochemistry may include sulfuric acid, hydrogen sulfide, and other nasties. Localized attack and pitting are the result.
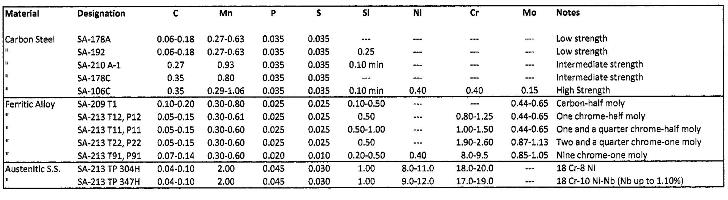
The universal material for condensate/feedwater piping and boiler waterwall tubes is mild carbon steel, as this material often offers the best combination of strength and price. A transition from the mild steels to higher-strength alloys, including the ferritic and austenitic steels, is needed in superheaters and reheaters where temperatures are higher and the density of the cooling medium is much lower. The table outlines the chemical composition of the most common materials employed for steam generator fabrication.
(Click to enlarge.) Common steam generator materials. Adapted from Ref. 1
In considering materials for condenser and feedwater heater tubes, current practice calls for all-ferrous alloys, usually stainless steel. Some older power-generating units still have heaters and/or condensers with copper alloy tubes. Most common are Admiralty metal (70% copper, 29% zinc, and 1% other alloys, primarily arsenic or phosphorous), and the copper-nickel alloys, notably 90-10 and 70-30.
Turbine rotors and blades are composed of high alloy steels. Typical for turbine blades is 403 SS, which contains 12% to 13% chromium. A popular material for modern supercritical plant turbine blades is type 422, 12 Cr ferritic steel. HP turbine rotors are often ferritic steels containing 1% to 13% chromium and some nickel, molybdenum, and vanadium.
Alloying Elements in Steel
At first glance, the numerous elements that make up the steels outlined in the table may be bewildering. Most are alloying elements to improve various properties (toughness, resistance to heat, and so on) of the steel, while a few are impurities whose content must be carefully controlled.
Carbon: Carbon is the alloying element that defines steel, as opposed to plain iron. As stated in Reference 2, it is the “most important alloying element in steel.” Carbon increases the tensile and yield strengths of steel, although it also lowers the ductility and toughness. As is evident in the table, a relatively small percentage of carbon is necessary, and indeed will dissolve in steel, for alloying purposes. Above about 2% concentration, the element forms carbon nodules in the metal; these are the cast irons, whose ductility and strength decrease with increasing carbon content.
Chromium: Chromium is the element that in 12% concentration or greater imparts the “stainless” quality to stainless steels. The chromium causes steel to form a layer of chromium oxide that protects the steel from its environment (but with cautions to that chemistry as will be outlined later). However, even at lower concentrations than 12% chromium benefits also may be derived, including greater strength and toughness, and improved resistance to creep and oxidation at high temperatures. (Creep is the deformation of steel at elevated temperatures.) Also, even small amounts of chromium provide increased resistance of steel to flow-accelerated corrosion (FAC). [3] Both single-phase and two-phase FAC have plagued many steam generating systems where some failures have caused fatalities.
Nickel: Nickel improves toughness and in certain cases corrosion resistance of steel. But, it is most important when alloyed at 8% concentration or higher, as at this concentration and above, nickel causes the steel to assume the austenitic structure.
A brief discussion of the two most common steel crystalline structures will aid in understanding. When metals are fabricated from molten material, the crystalline structures that form grow into grains as the material solidifies.
Illustration of metal grains. Source: Wikipedia.Grain size may vary depending on the metal or alloy and fabrication methods, for example, fast cooling, slow cooling, subsequent heat treatment. We will examine grain behavior later with regard to some special corrosion issues. For example, grain boundaries often serve as corrosion sites.
The crystalline structure of the metal within grains is also important. For the mild steels and even those that contain significant chromium, but without nickel, each unit cell of the crystal has a body-centered cubic structure, as shown.
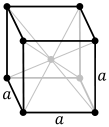
The body-centered cubic structure. Note the atom centered within the cell. Source: Wikipedia.These steels are defined as ferritic. If the steel is heated to a high temperature, this structure morphs into a face-centered cubic structure known as austenite.
In some cases, austenite offers better mechanical properties than ferrite. The addition of 8% or greater nickel to steel as an alloying element allows the steel to remain as austenite at room temperatures and below.
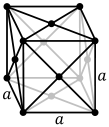
The face-centered cubic structure. Source: Wikipedia.The table may now become more understandable with regard to the crystal structure of each of the steels. A third structure, martensite, can also be produced by special treatment, but it will not be considered here. However, note that a relatively new type of steel, the duplex alloys, are being used in some applications. These consist of a combination of ferrite and austenite. The duplex alloys offer some advantages, but at least one spectacular failure mechanism is known, in an air pollution control application, which will be outlined in a later article.
Molybdenum: Molybdenum increases steel’s strength and resistance to wear, among other benefits, but also contributes to high temperature strength.
Others: Elements like silicon and manganese can help protect steels against oxidation and problems related with residual sulfur in the steel, respectively. Phosphorus in low concentrations can increase steel hardness, but becomes troublesome in higher concentrations than those shown in the table. Other trace elements that may be beneficial, depending upon the alloy, include aluminum, boron, columbium, copper, nitrogen, niobium, and tungsten.
Some Mechanical Steel Failure Methods
The components of a high-pressure steam generator are subject to a number of mechanical stresses, including tensile, compression, and others. These often occur at attachments such as supporting infrastructure and tubing connections at headers, drums, and similar locations. Stressed locations are often greatly influenced by the high temperatures during normal operation and cyclic issues when units are reduced in load or come off-line, as has become much more common in recent years.
Several stresses will come into focus in later corrosion discussions. For example, some components that cycle frequently may fail from simple fatigue. Tube attachments are classic locations for fatigue. Corrosion fatigue, as the name implies, involves structural weakening by fatigue that then allows corrosive agents to accumulate in the weakened area, usually a small crack, and then increase the corrosion rate. Stress corrosion cracking (SCC) can occur in components; turbine blades are a prime example, in which normal operation induces stresses that allow corrosion to occur.
Another common phenomenon in steam generators is the deformation known as creep. Materials begin to lose strength at elevated temperatures; the ultimate example may be if the metal is taken to its melting point. But even at temperatures well below the melting point, the combination of heat and stress will cause metals to deform.
Very common to high-pressure steam units, and especially in superheaters and reheaters, is long-term creep. Over time, the material may deform or elongate to the point of failure. Corrosion often may play a part in long-term creep, particularly if corrosion products inhibit heat transfer. At the opposite extreme, if corrosion or some other mechanism causes a partial or full blockage of fluid flow in high-temperature water or steam-bearing tubes, short-term overheat may be the result.

Rapid tube failure. Note the thin-lipped structure at the failure. Source: ChemTreat.Such dramatic failures may be corrosion/chemistry related, but also may be induced by operational upsets. In coal-fired power plants, firing a unit too rapidly is one possibility. Another, which has occurred over the years, is neglecting drum level during operation and allowing portions of waterwall tubes to go dry.
In Part 3, we will examine primary corrosion mechanisms and their control in greater detail. These issues are too-often overlooked, sometimes to the point of a catastrophic failure.
Materials may range from mild steel in condensate/feedwater systems and waterwall tubes to high-alloy steels in superheater/reheaters and turbines. Alloying elements obviously have a strong impact on corrosion mechanisms that can affect the various steels. In Part 3 we will examine corrosion issues, and include a discussion of methods of control. Corrosion is quite a complex science, with steam generation corrosion being just one part of a huge mosaic.
Mild Carbon Steel Corrosion and Control - Feedwater
Iron is an amphoteric metal, meaning that both low and very high pH solutions will cause corrosion. Part 1 of this series outlined the basic corrosion mechanism in acid. At the temperatures common to the condensate/feedwater system and steam generator, general corrosion is minimized at a mildly basic pH.
For typical heat recovery steam generators (HRSGs) at combined-cycle plants, the recommended feedwater pH range is 9.6 to 10 (as measured at 25oC). The most common chemical used for feedwater pH control is ammonia (NH3), a weak base that generates hydroxyl ion (OH-).
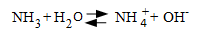
Because feedwater is pure (or always should be), a common method to control ammonia feed is from specific conductivity (S.C.) readings, as in pure water a direct correlation exists between S.C. and pH, with S.C. being much easier to measure.
Beyond pH, however, another issue has arisen regarding flow accelerated corrosion (FAC). A future article will cover that topic, but it is worth mentioning here that in the condensate/feedwater systems of modern HRSGs (virtually none of which contain copper alloys) a small amount of dissolved oxygen (5 to 10 ppb in the feedwater) is required to generate the proper protective oxide surface on carbon steel. As we will review in that future article, FAC is an extremely serious issue.
From a materials aspect regarding FAC control, fabrication of FAC-susceptible components with P11/T11 or P22/T22 (1¼ and 2¼ chromium concentration, respectively) should be a standard consideration for all new HRSGs.
Yet another important subject, which requires a separate article for full discussion, is steam generator corrosion control when the unit is off-line. In these cases, oxygen is an enemy, and air in-leakage to cold units standing full of water can cause damaging corrosion.
A technology for corrosion protection that has been known for decades and is now re-emerging, is filming amine use. Amines are organic compounds with one or more ammonia groups attached. (Some small chain amines, known as neutralizing amines, are used in place of or along with ammonia as pH-conditioning agents.)
The amine groups and perhaps other functional groups on the chain attach to metals, while the hydrophilic organic chain establishes a barrier to water. In recent years, some positive results have been seen in full-scale tests of these products. Potential drawbacks still remain, including organic breakdown in high-temperature steam. Organizations such as the Electric Power Research Institute (EPRI) continue research into these products.
Boiler Water Corrosion Control
The ammonia (or perhaps neutralizing amine) used for condensate/feedwater conditioning can provide enough alkalinity to also protect the steam generator proper, as long as impurity intrusion is prevented. But condenser tube leaks will introduce a variety of contaminants to the condensate and the steam generator, including scale-forming ions and also chloride and sulfate, among others. The latter, and chloride in particular, can generate acids that quickly consume ammonia and leave the steam generator in a vulnerable condition.
So, basically from the advent of high-pressure power production, most steam generators (and here we are only examining drum-type boilers and HRSGs) are treated with an additional pH-conditioning agent. Most common has been tri-sodium phosphate (Na3PO4), which generates alkalinity as follows:
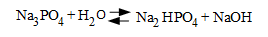
A common recommended pH range for high-pressure HRSG evaporators is 9.2 to 9.7. [1]
Phosphate will also react with hardness ions to prevent them from forming hard scale in the steam generator, but unfortunately, TSP has some drawbacks that limit its effectiveness. One of the most notable is its reverse solubility at high temperatures.
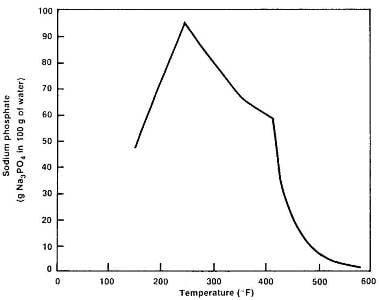
Tri-sodium phosphate solubility vs. temperature. Source: Ref. 2, original source EPRI.The compound cannot be dosed liberally, as most of it precipitates directly on boiler internals. Compounding this issue is that the TSP can react directly with the boiler tube material to form iron-sodium phosphate compounds. For these reasons, chemists at some plants have adopted straight caustic (NaOH) feed as the method to establish boiler water alkalinity. However, due to iron’s amphoteric nature, the caustic concentration must be limited to a maximum of 1 part-per-million (ppm).
At this point, another factor must be considered. Even in systems with good feedwater chemistry, iron oxide corrosion products still are generated and travel to the steam generator. At the high boiler temperatures ,the particulates precipitate, generally on the hot side of the tubes. Deposition sets up porous deposits, where water can penetrate the deposits through various channels.

An illustration of wick boiling.As the water approaches the tube surface, temperatures increase. The water boils off, leaving impurities behind (wick boiling). The contaminants can concentrate to very high levels. One of the most frightening reactions possible is shown in the following equation:
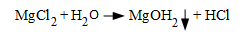
As can be seen, a product of this reaction is hydrochloric acid. While HCl may cause corrosion in and of itself, the compound will concentrate under deposits, where the reaction of the acid with iron generates hydrogen, which in turn can lead to hydrogen damage of the tubes.
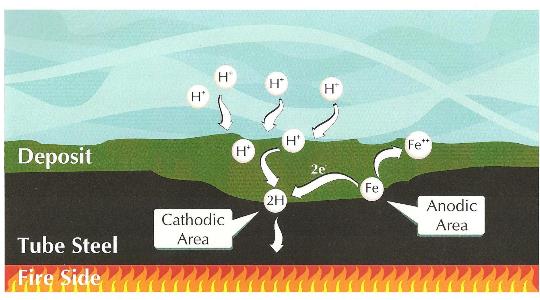
Under-deposit acid formation. Illustration courtesy of Ray Post, ChemTreat.In this mechanism, atomic hydrogen penetrates into the metal where it reacts with carbon atoms in the steel to generate methane (CH4). Formation of the gaseous methane and hydrogen molecules causes cracking in the steel, greatly compromising its strength. Hydrogen damage is troublesome because it cannot be easily detected. After hydrogen damage has occurred, plant staff may replace tubes only to find that other tubes continue to rupture. [3]

A tube failure due to hydrogen damage. Notice the thick-lipped fracture, indicative of failure with little metal loss. Photo courtesy of ChemTreat.For many decades, hydrogen damage has been at or near the top of the list of high-pressure steam generator corrosion mechanisms. What power plant personnel often fail to recognize is that even small condenser tube leaks (or other impurity ingress), if they are chronic, can allow repetitive under-deposit corrosion and hydrogen damage.
Under-deposit corrosion is not limited to low pH conditions. Per the wick boiling example outlined above, another compound that can concentrate within deposits is caustic. Concentrations may rise to levels many times that in the bulk boiler water. The concentrated NaOH attacks the boiler metal and its protective magnetite (Fe3O4) layer via the following reactions:
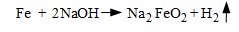
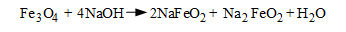
The potential for under-deposit caustic gouging is the primary reason why the free caustic concentration in the boiler water is typically limited to 1 ppm.
Key Takeaways
The reader will have observed a number of important items in this discussion, but several takeaways should be emphasized.
• The amphoteric nature of iron requires pH control within a rather narrow window.
• Deposits that accumulate within the steam generator greatly influence the corrosion potential due to their ability to allow concentration of impurities. That is why regular chemical cleaning is important, although this can be a difficult task in HRSGs with the complex evaporator tubing.
• Proper feedwater chemistry control is required not only to prevent FAC, but also to minimize carryover of particulates to the steam generator.
• Units should not be operated with condenser tube leaks unless the condensate system is equipped with a condensate polisher.
A Note on Steam Chemistry
Space did not permit a discussion about steam chemistry, but it is highly important. In fact, many of the guidelines for boiler water chemistry have developed around the prevention of impurity carryover to the steam system and turbine. The corrosion mechanisms that certain impurities can induce include pitting, stress corrosion cracking, and corrosion fatigue.
References
1. Comprehensive Cycle Chemistry Guidelines for Combined Cycle/Heat Recovery Steam Generators (HRSGs). EPRI, Palo Alto, CA: 2013. 3002001381.
2. Buecker, B., and S. Shulder, “Critical Water/Steam Chemistry Concepts for HRSGs”; webinar for Energy-Tech University, Oct. 25-26, 2016.
3. B. Buecker, “Condenser Chemistry and Performance Monitoring: A Critical Necessity for Reliable Steam Plant Operation”; paper IWC 99-10 from the Proceedings of the 60th Annual International Water Conference, October 18-20, 1999, Pittsburgh, Penn.