At many large industrial plants (and a large section of the electric power industry), steam is the lifeblood of the plant. Preparing high-purity water and converting it into high-purity steam are mandatory for reliable plant performance. Ubiquitous at all of these plants are closed cooling water (CCW) systems for temperature control of pump bearings, auxiliary heat exchangers and other plant components.
CCW systems often reside in the background and can be forgotten when it comes to monitoring and treatment upkeep. Often, only when a system fails do plant operators and technical personnel take notice. One issue that can compound corrosion control techniques in particular is that a number of different metals may be present in the system. Piping may be carbon steel, but heat exchanger tubes may be a copper-alloy material or even stainless steel. Other metals such as aluminum also might be present.
Fundamental Principles of Corrosion
Metal corrosion always involves an electrochemical process, although in the case of erosion-corrosion, mechanical influences are also present. Although many different types of corrosion have been discovered, and some have not been completely investigated, the following example outlines some core fundamental concepts. Consider a laboratory experiment that involves immersing a steel bar into a hydrochloric acid (HCl) solution. Within short order, corrosion spots will become plainly visible on the steel. The figure illustrates the corrosion mechanism.

Fig. 1 Basic corrosion cell.Three steps explain the basic process:
1. Each iron atom at the corrosion site gives up two electrons (oxidizes), and thus transforms from a zero oxidation state to a +2 oxidation state (Fe0 ® Fe+2 + 2e-). The Fe+2 (ferrous) ions migrate into the solution. This site is known as the anode.
2. The electrons flow through the metal to another site where they react with the hydrogen ions of the acid (reduction) to produce hydrogen gas (2H+ + 2e- ® H2^ ). This reaction occurs at the cathode.
3. The anions from the acid, chloride ions (Cl-), and the ferrous ions migrate through solution to produce solvated ferrous chloride (FeCl2).
This example shows that an electrical circuit is completed. Without this electrical circuit, corrosion does not occur.
In plant cooling water systems, a different cathodic reaction is typical, and involves dissolved oxygen in the water rather than acid.
O2 + 2H2O + 4e- ® 4OH-
Oxygen reduction occurs at the cathode, and the hydroxyl ions (OH-) that are produced combine with iron to form ferrous hydroxide, [Fe(OH)2], which further oxides to ferric hydroxide precipitate [Fe(OH)3]. De-hydrolysis of ferric hydroxide produces Fe2O3, which we know as rust. Dissolved oxygen in cooling water can cause pitting, an insidious corrosion mechanism.
Many types of cooling water corrosion are possible, including general corrosion, pitting, crevice corrosion, stress corrosion cracking, galvanic corrosion and others. Many articles and books have been written on these mechanisms, so this article will focus on current methods to minimize corrosion.
Preventing Corrosion in CCWs
In an open cooling water system, the exchange of process water for fresh makeup continuously brings corrosive agents to metal surfaces. But, in an ideal closed cooling water system, any corrosive compounds that are in the initial charge of water will be consumed and will not be replenished. CCWs are never completely closed, and process leakage requires some makeup addition. With this makeup comes the need for chemical treatment to minimize the steel corrosion reactions outlined above, and others as we will examine. Importantly, if corrosion is not controlled and leaks develop, makeup increases which only exacerbates the problems by increasing the quantity of corrosives that are introduced to the system.
Commonly, condensate or other high-purity water is used for filling and subsequent makeup in CCWs. This eliminates the possibility of scale formation that could occur if raw or service water was used as the source. The most common example is calcium carbonate (CaCO3) scaling,where, as the temperature of the water increases, the following reaction occurs:
Ca+2 + 2HCO3- + heat → CaCO3↓ + CO2 + H2O
This deposition mechanism has plagued heat exchangers since the dawn of the Industrial Age. Other scale-forming compounds are possible, including sulfates and silicates.
For systems with deionized water as the makeup, these scale-formation issues need not concern us now. Look again at the earlier figure. Corrosion can be controlled either by inhibiting the cathodic or anodic reaction. In open cooling water systems, typical chemistry is designed to inhibit both. But, in closed cooling water systems, anodic inhibition is the typical method.
Sodium di-chromate (Na2Cr2O7) formerly was the most common chemical feed to both open and closed cooling systems. The di-chromate would form a protective oxide layer at anodic sites that would eventually cover the entire carbon steel surface. In fact, the film has been described as a pseudo-stainless steel due to its chromium content. The chemistry was straightforward to control and provided excellent results. However, several decades ago chromate treatment was banned due to the toxicity of hexavalent chromium (Cr6+). The two most common replacements have been nitrite (NO2-) and molybdate (MoO4-), usually supplied in the sodium form. These compounds also form an oxide film at anodic sites, inhibiting reaction. Typical guidelines for proper control are as follows: [1]
Nitrite
· Concentration range: 500 to 1,500 ppm as NO2-
· pH range: 8.5 to 11.0 (with an azole for copper alloy protection, see below)
Molybdate
· Concentration range: 200 to 1,000 ppm as NO2-
· pH range: 9.0 to 11.0 (with an azole for copper alloy protection)
At times, the two chemicals are used in combination to provide enhanced benefits. Of note is the mildly alkaline pH range at which these treatments are most effective. Also, nitrite functions well without the presence of any dissolved oxygen.
While these compounds when properly used and monitored can provide excellent corrosion control in closed cooling water systems, a number of issues must be considered.
· Regular monitoring (common is on a weekly basis) is required to ensure that the concentration remains within the control range. If regular monitoring is not done and concentration drops too low, anodic corrosion sites will begin to appear. The relatively few anodes in a large cathodic field will lead to pitting and through-wall penetration, which is undesirable. It is better not to feed any inhibitor at all than allow pitting corrosion to develop.
· Excess system leakage (either due to corrosion failures or mechanical problems) requires increased makeup. In turn, this dilutes the chemistry and introduces additional corrosive agents. This can become a cascading problem.
· The compounds do not control microbiological growth, and some, such as nitrites and azoles, offer nutrients and food for microbes. (The author directly observed this phenomenon in an extensive closed cooling water system at an automobile assembly plant.) Microbes will foul systems and may also cause microbiologically induced corrosion (MIC). Close monitoring of microbiological activity may be required, with possible feed of a biocide to control the organisms.
· Some formulations, and particularly non-oxidizing biocides, may contain stabilizing compounds that contain chlorides. These can cause pitting in heat exchanger tubes made of stainless steel.
Copper Alloy Protection
Many auxiliary heat exchangers have copper-alloy tubes, primarily because copper is a good heat conductor. Common copper corrosion protection is built around an affinity for copper to bond with certain nitrogen compounds, although this tendency can at times be problematic. For example, copper alloys in solutions containing ammonia and oxygen will corrode quickly and severely. However, it is also this fact that allows corrosion protection. The figure outlines the structure of the first common corrosion inhibitor, tolyltriazole.
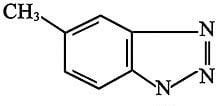
Fig. 2. Chemical structure of tolyltriazole.When this compound is added to the cooling water, the nitrogen atoms bond to copper while the organic ring provides a shield from the environment. Additional azoles have been developed that are better at resisting decomposition from oxidizing biocides, heat, or other factors, but the principle remains the same.
Although closed cooling water systems reside in the background at industrial power plants, they are critical for operation. A failure due to poor chemistry control, especially when preventative expenses are minimal, may cost a plant many times over in lost production. To establish a proper program, a full evaluation of system metallurgy, flow rates, and other variables is required, with attention paid to chemistry monitoring.
Reference
1. Closed Cooling Water Chemistry Guideline: Revision 1 to TR-107396, Closed Cooling Water Chemistry Guideline, EPRI, Palo Alto, Calif.: 2004. 1007820.
https://insights.globalspec.com/article/2265/don-t-neglect-closed-cooling-system-water-treatment