Many new power plants and industrial facilities continue to be constructed and placed into service around the world. Steam condensation is an important process at these plants, and many of them are equipped with cooling towers.
These towers and other portions of cooling system networks provide a superb environment for microbiological growth and fouling. Without proper microbiological control, organisms can form vast colonies that may lead to the following undesirable conditions.
- Reduced energy transfer in condensers and heat exchangers that can cost a plant hundreds of thousands to perhaps millions of dollars per year.
- Formation of microbiological slime that leads to under-deposit corrosion and failures in heat exchangers. Again, costs can be quite high due to loss of production and materials replacement needs.
- Flow restrictions in cooling tower film fill that reduce plant efficiency. Partial collapse of cooling towers has occurred due to accumulation of microbiological deposits, whose slime layers also collect silt from the makeup water and air passing through the tower.
- Growth of pathogenic organisms such as Legionella bacteria, which continues to cause fatalities around the world.
Indeed, reports from the U.S. Centers for Disease Control show that the bacteria behind Legionella can take root in a myriad of water sources, including cooling towers. An investigation pinpointed cooling towers used for air conditioning as the source of more than 100 illnesses and 12 deaths in the South Bronx this summer.
Microbiologically polluted heat exchanger tubes and cooling tower film fill. These deposits severely restrict heat transfer, fluid flow and may lead to severe under-deposit corrosion. Source: Post R. and B. Buecker, “Power Plant Cooling Water Fundamentals”; pre-conference seminar for the 33rd Annual Electric Utility Chemistry Workshop, June 13-15, 2013, Champaign, Ill.An obvious but challenging aspect of cooling tower operation is keeping the tower, condenser and other system components free of microbiological fouling, scaling and solids deposition. Even with fresh water from a lake or river as makeup, cooling system chemistry control requires diligence and good planning.
Cooling systems provide an ideal environment—warm and wet—for microbes. Bacteria will grow in condensers and cooling tower fill, fungi on and in cooling tower, wood and algae on wet cooling tower components exposed to sunlight. Rigorous biocide treatment is essential to maintain cooling system performance and integrity.
Know Your Bacteria
Bacteria are separated into the following three categories:
- Aerobic: Utilize oxygen in the metabolic process.
- Anaerobic: Live in oxygen-free environments and use other sources, for example, sulfates, nitrates or other donors for their energy supply.
- Facultative: Can live in aerobic or anaerobic environments.
A problem with microbes, particularly bacteria, is that once they settle on a surface, the organisms secrete a polysaccharide layer for protection. This film then collects silt from the water, thus growing even thicker and further reducing heat transfer. Even though the bacteria at the surface may be aerobic, the secretion layer allows anaerobic bacteria underneath to flourish.
These bacteria can generate acids and other harmful compounds that directly attack the metal. Microbial deposits also establish concentration cells, where the lack of oxygen underneath the deposit causes the locations to become anodic to other areas of exposed metal. Pitting is often a result, which can cause tube failure well before the expected lifetime of the material.
Fungi will attack cooling tower wood in an irreversible manner, which can lead to structural failure. Algae will pollute cooling tower spray decks, potentially leading to reduced performance and unsafe working locations.
Treatment Options
The core of most microbiological treatment programs is the feed of an oxidizing biocide to kill organisms before they can settle on condenser tube walls, cooling tower fill and other locations. Chlorine was the workhorse for many years, where when gaseous chlorine is added to water the following reaction occurs:
Cl2 + H2O <> HOCl + HCl
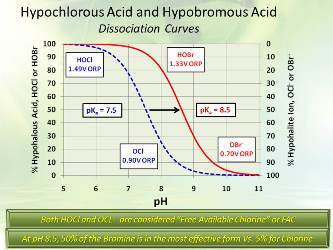
HOCl, hypochlorous acid, is the killing agent. The functionality and killing power of this compound are affected by pH due to the equilibrium nature of HOCl in water.
HOCl <> H+ + OCl-
OCl- is a much weaker biocide than HOCl, probably due to the fact that the charge on the OCl- ion does not allow it to penetrate cell walls. The killing efficiency of chlorine dramatically declines as the pH goes above 7.5. Thus, for the common alkaline scale/corrosion treatment programs, chlorine chemistry may not be efficient.
Chlorine demand is further affected by ammonia or amines in the water, which react irreversibly to form the much less potent chloramines. Due to
safety concerns, liquid bleach (NaOCl) feed has replaced gaseous chlorine at many facilities.
Hypochlorous acid and hypobromous acid dissociation curvesA popular alternative is bromine chemistry, where a chlorine oxidizer and a bromide salt, typically sodium bromide (NaBr), are blended in a makeup water stream and injected into the cooling water. The chemistry produces hypobromous acid (HOBr), which has similar killing powers to HOCl, but functions more effectively at alkaline pH.
Chlorine dioxide (ClO2) is becoming more popular for several reasons. Its killing power is not affected by pH, the chemical does not react with ammonia and it does not form halogenated organic compounds. Also, chlorine dioxide is more effective in attacking established bio-deposits. ClO2 is unstable and must be generated on-site. Past methods were expensive and unsafe, but much improved technology is now available.
Yet another chemistry that has been used in some cases is a feed of specially generated monochloramine (NH4Cl). Although it is a weaker oxidizing agent than chlorine, it appears to do a good job at penetrating biological slime layers to kill the organisms underneath.
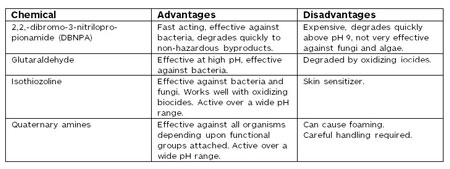
An additional method to help control microbes is a supplemental feed of a non-oxidizing biocide. Typically, feed is needed on a temporary but regular basis, perhaps once a week. The table outlines some of the properties of the most common non-oxidizers.
Properties of common non-oxidizers. Source: Author's constructCareful evaluation of the microbial species in the cooling water is necessary to determine the most effective biocides. None of these chemicals should be used or even tested without approval from the appropriate regulating agency.
As with all chemicals, safety is a critical issue when handling the non-oxidizers. Adherence to all handling guidelines and use of proper personal protective equipment is a must. Beware that many of these chemicals will attack human cells as well as those of microbes.